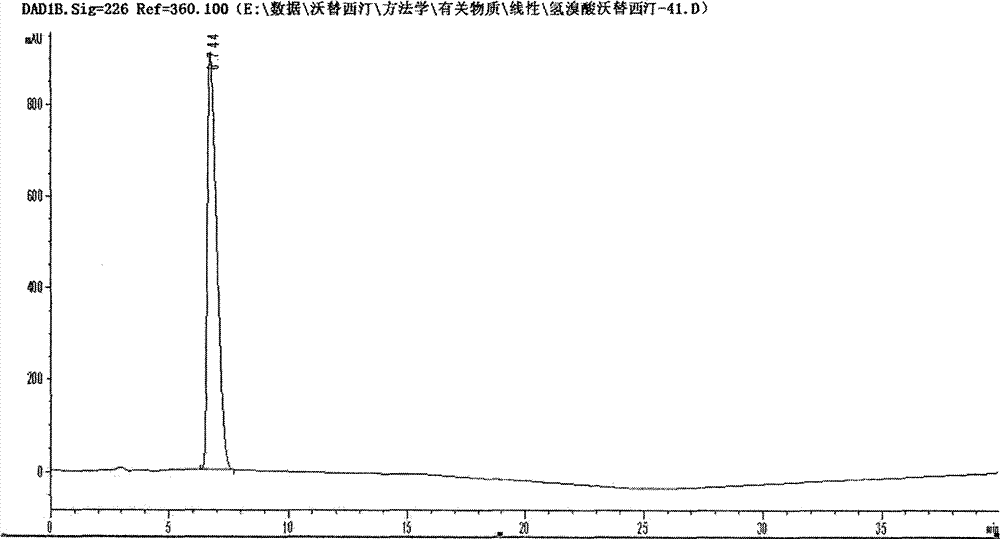
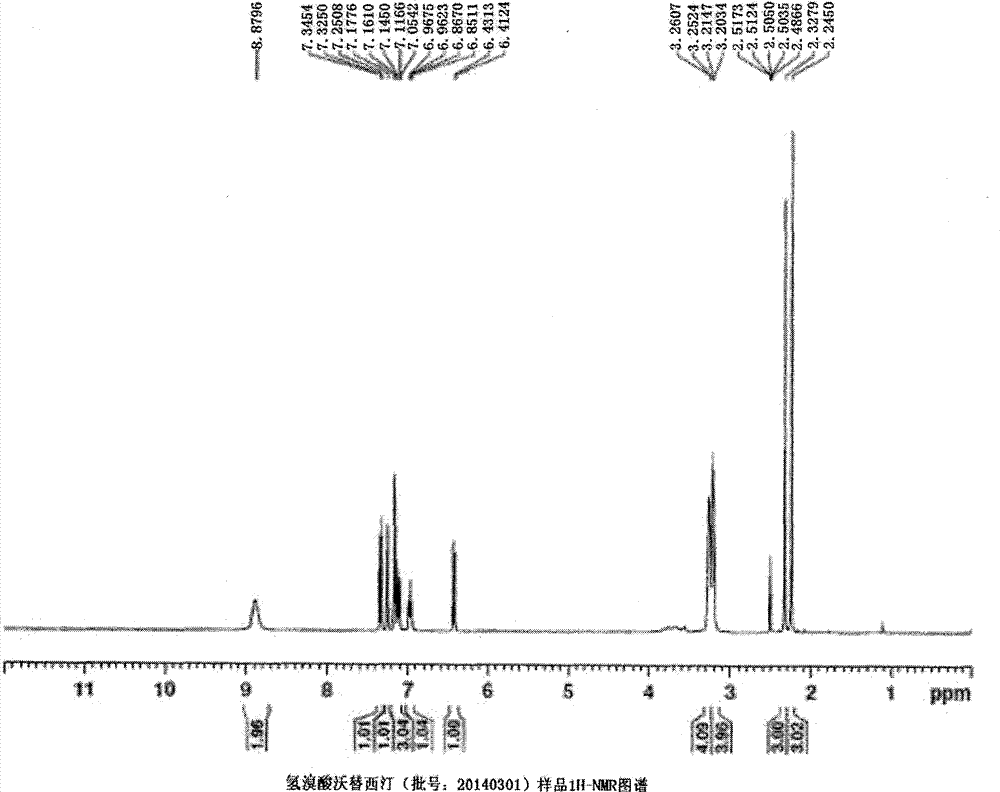
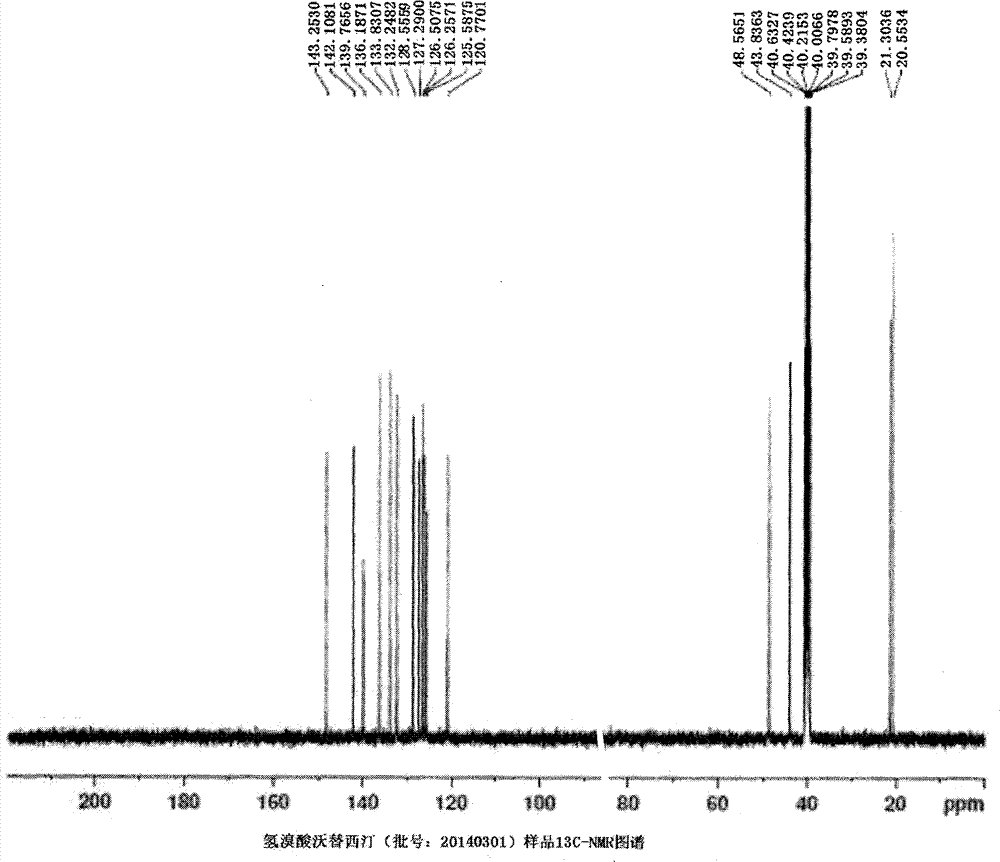
Preparation method of high-purity vortioxetine hydrobromide
A technology of vortioxetine hydrobromide and vortioxetine, which is applied in the field of preparation of high-purity vortioxetine hydrobromide, can solve the problem of high purity of vortioxetine hydrobromide and the production purity of vortioxetine Low cost, high cost, etc., to achieve the effects of easy industrial production, high product yield, and mild process reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: This embodiment provides a kind of preparation method of high-purity vortioxetine hydrobromide, and concrete steps are as follows:
[0025] Step 1: Synthesis of 2-(2,4-dimethylphenylsulfanyl) chlorobenzene:
[0026]
[0027] Get a 250ml reaction bottle, feed nitrogen into the bottle, get 20g (0.16mol) of 2-chlorophenol (formula I) and 21.5g (0.16mol) of 2,4-dimethylthiophenol (formula II) and add to the reaction Add 135.3g of ethyl acetate, 0.9g (0.016mol) of nickel nanopowder, 39.4g (0.48mol) of sodium isopropoxide and 10g of anhydrous sodium sulfate into the bottle, stir at room temperature for 20min, raise the temperature to 50°C, and stir for 8h , TLC to monitor the reaction process, after the reaction is over, stop heating, and filter the reaction solution after it drops to room temperature, wash the filtrate 3 times, 50ml each time, take the organic phase, add anhydrous sodium sulfate to dry overnight, filter, and the filtrate is rotated The solv...
Embodiment 2
[0035] Embodiment 2: This embodiment provides another kind of preparation method of high-purity vortioxetine hydrobromide, and concrete steps are as follows:
[0036] Step 1: Synthesis of 2-(2,4-dimethylphenylsulfanyl) chlorobenzene:
[0037]
[0038] Get a 500ml reaction bottle, feed nitrogen into the bottle, get 30g (0.24mol) of 2-chlorophenol (formula I) and 32.25g (0.24mol) of 2,4-dimethylthiophenol (formula II) into the reaction Add 202.95g of ethyl acetate, 1.35g (0.024mol) of nickel nanopowder, 59.1g (0.72mol) of sodium isopropoxide and 15g of anhydrous sodium sulfate into the bottle, stir at room temperature for 23min, raise the temperature to 55°C, and stir for 9h , TLC to monitor the reaction process, after the reaction is over, stop heating, and filter the reaction solution after it drops to room temperature (20-25°C), wash the filtrate with water 3 times, 60ml each time, take the organic phase, add anhydrous sodium sulfate to dry overnight , filtered, and the f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com