Photoelectric material and preparation method thereof and application to organic electronic device
A technology of optoelectronic materials and synthesis methods, which is applied in the fields of electric solid state devices, photovoltaic power generation, electrical components, etc., can solve the problems of high cost and complicated preparation process of optoelectronic materials, and achieve the effects of low manufacturing cost, easy purification and good yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
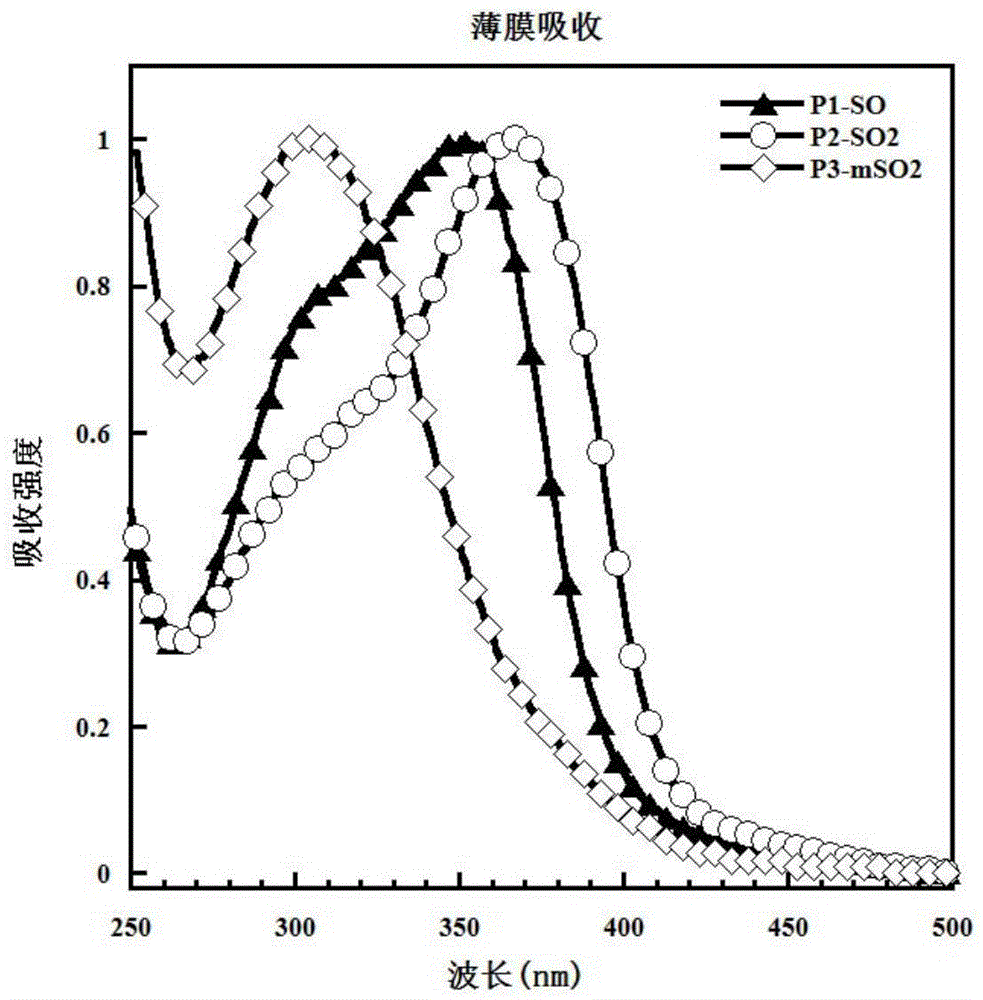
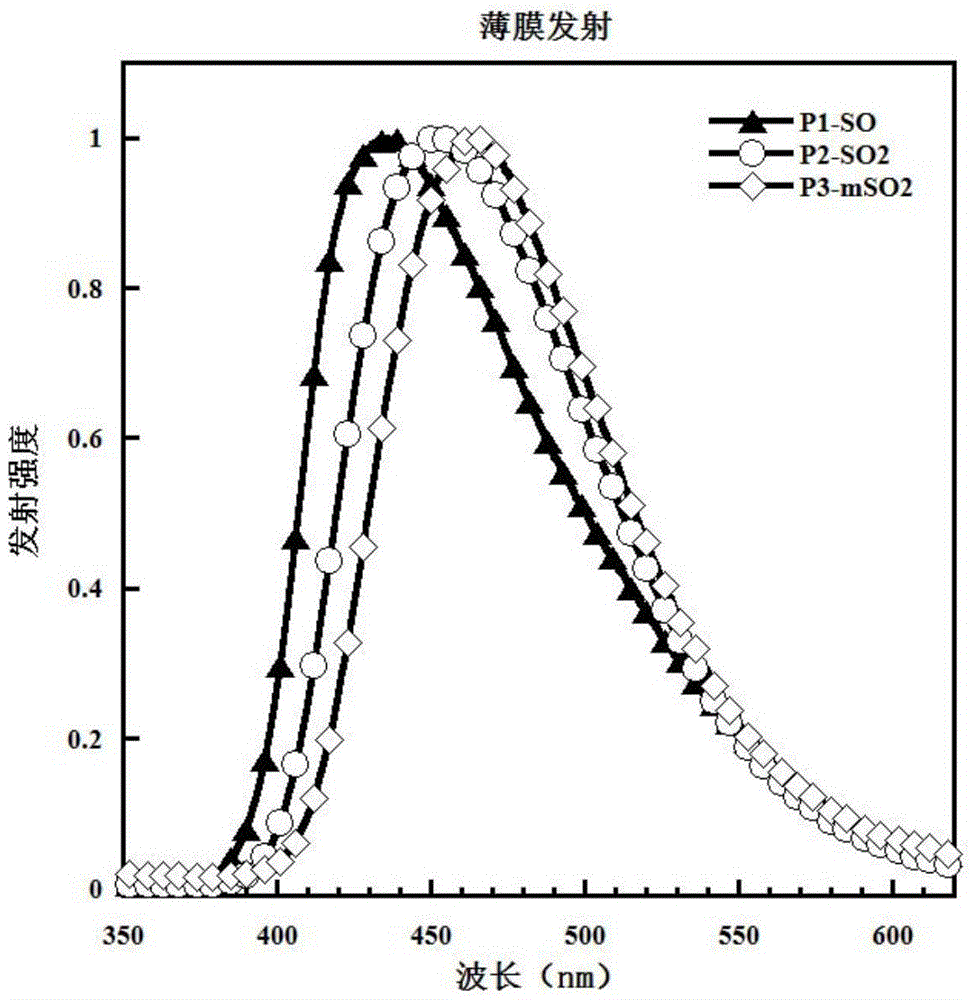
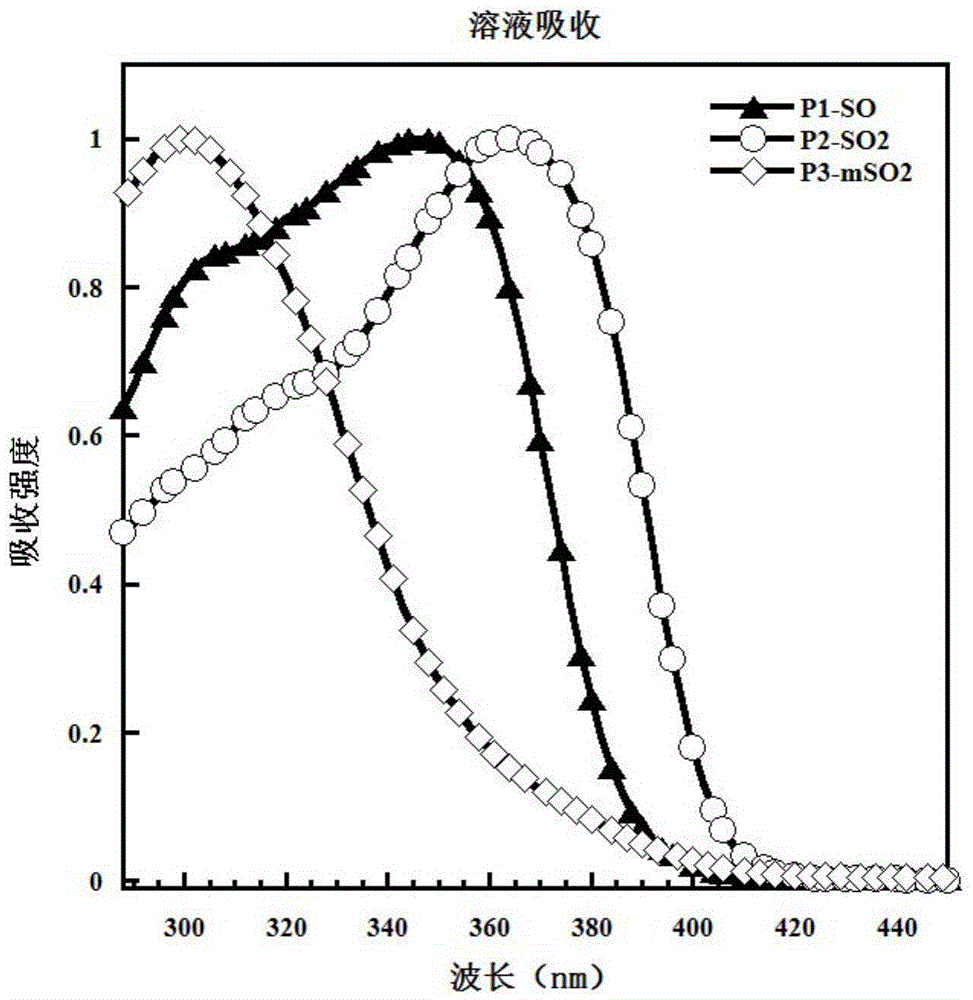
[0052] Example 1: Synthesis of P1-SO
[0053] (1) Synthesis of dibromodiphenyl sulfoxide
[0054] Diphenylsulfide (2.34mL, 20mmol) was dissolved in CH 2 Cl 2 and H 2 O (1:1, 150mL) mixed solvent, add 30% H 2 o 2 (0.86mL, 36mmol) and liquid bromine (4.1mL, 80mmol), and then the reaction solution was stirred continuously at room temperature for 6h. After the reaction was completed, excess liquid bromine was removed with saturated sodium sulfite solution, and then extracted with water and dichloromethane , after the organic phase was dried with anhydrous magnesium sulfate, spin-dried in vacuum in a rotary evaporator to obtain a crude product, then carried out column chromatographic separation and purification, utilized sherwood oil as an eluent, and finally carried out recrystallization with sherwood oil to obtain 5.88g ( 86%) white solid, 1 H-NMR (500MHz, CDCl 3 ): δ (ppm) = 7.59-7.63 (d, 4H), 7.48-7.52 (d, 4H), due to the good symmetry of this structure, it shows two typ...
Embodiment 2
[0062] Example 2: P2-SO 2 Synthesis
[0063] (1) Synthesis of Dibromodiphenylsulfone
[0064] Dibromodiphenyl sulfide (11.7253g, 34.1mmol), 30% H 2 o 2 (150mL) and HOAc (150mL) were added into a single-necked bottle and heated to reflux in an oil bath overnight. After the reaction was completed, filter and recrystallize with ethanol to obtain pure product dibromodiphenyl sulfone with a yield of 88.5%. 1 H-NMR (500MHz, CDCl 3 ): δ (ppm) = 7.77-7.79 (d, 4H), 7.64-7.67 (d, 4H), due to the good symmetry of this structure, it shows two types of hydrogen, and the nuclear magnetic resonance is in good agreement with it, that is, the product is obtained Dibromodiphenyl sulfone; preparation process is shown in the following formula (6):
[0065] Formula (6):
[0066] (2) With reference to the step (2) of Example 1, 4,4'-to-octyloxyanilinodiphenylsulfone was synthesized, a pale yellow solid with a yield of 88%, 1 H-NMR (500MHz, DMSO-d 6):δ(ppm)=8.56(s,2H),7.57-7.60(d,4H),7.06-...
Embodiment 3
[0068] Example 3: P3-mSO 2 Synthesis.
[0069] (1) Synthesis of meta-dibromodiphenyl sulfone.
[0070] Add NBS (17.8g, 100mmol) in batches to a solution of diphenylsulfone (10.9g, 50.0mmol) in sulfuric acid (50mL), and stir the reaction solution at 100°C for 2h. Poured into 100mL of ice water to obtain a white precipitate, which was filtered and recrystallized with ethanol to obtain the pure product m-dibromodiphenylsulfone with a yield of 58.8%. 1 H-NMR (500MHz, CDCl 3 ): δ (ppm) = 8.07 (s, 2H), 7.86-7.89 (d, 2H), 7.71-7.74 (d, 2H), 7.39-7.43 (m, 2H), the hydrogen at the chemical shift of 8.07, corresponding to The most important thing is the left and right hydrogens sandwiched between the sulfone group and bromine, and the nuclear magnetic resonance is very good, that is, the product m-dibromodiphenyl sulfone is obtained; the preparation process is shown in the following formula (8).
[0071] Formula (8):
[0072] (2) With reference to the step (2) of Example 1, 3,3'-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com