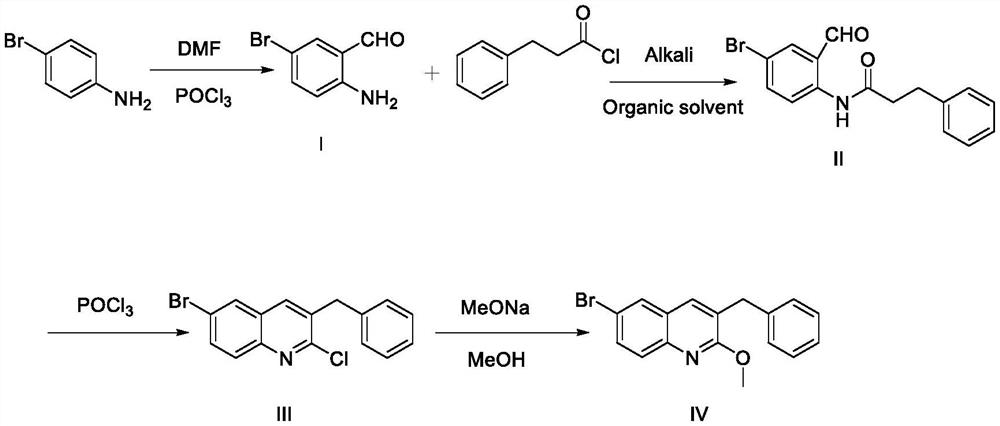
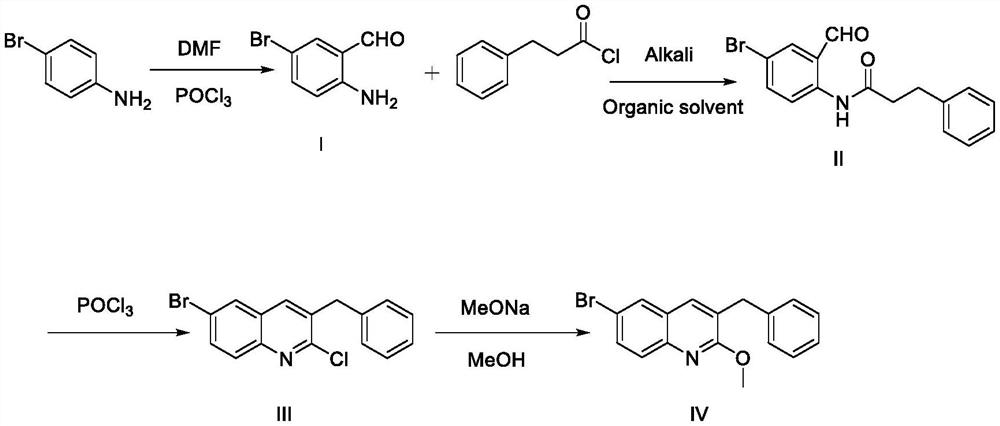
Synthesis method of 3-benzyl-6-bromo-2-methoxyquinoline
A technology of methoxyquinoline and synthetic method, which is applied in the field of drug synthesis, can solve the problems of unfavorable large-scale production operation, low yield, and low purity of the reaction system, and achieve the effect of clear reaction process and increased yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 3-benzyl-6-bromo-2-methoxyquinoline, including the following four steps:
[0031] Synthesis of Compound (I)
[0032] Phosphorus oxychloride (169 g, 1.1 mol) was added dropwise to a solution of N,N-dimethylformamide (80 g, 1.1 mol) in acetonitrile (1000 ml) at 10 °C, and the reaction was stirred at 10 to 20 °C for 0.5 h, add p-bromoaniline (172 g, 1 mol) at 10-20°C, heat up to 80°C for 4-6h after the addition, control the reaction in HPLC, complete the reaction, cool down to 0-5°C, slowly add aqueous sodium hydroxide solution , control the internal temperature below 20 degrees Celsius, add and stir for 0.5h, filter out the solid with a Buchner funnel, rinse with water twice, and dry to obtain 17.4g of off-white solid with a purity of 97.3% and a yield of 86.2%.
[0033] Synthesis of Compound (II)
[0034] Triethylamine (105 g, 1.1 mol) and compound I (200 g, 1.0 mol) were successively added to dichloromethane (1000 ml) at 25 °C, cooled to 0-5 °C in an ice bath, and phe...
Embodiment 2
[0040] 3-benzyl-6-bromo-2-methoxyquinoline, including the following four steps:
[0041] Synthesis of Compound (I)
[0042] Phosphorus oxychloride (30.7 g, 0.2 mol) was added dropwise to a solution of N,N-dimethylformamide (14.6 g, 0.2 mol) in acetonitrile (100 ml) at 10 °C, and stirred at 10 to 20 °C after the addition was complete. After reaction for 0.5h, p-bromoaniline (17.2g, 0.1mol) was added at 10-20°C, the temperature was raised to 80°C for 4-6h, the reaction was controlled by HPLC, the reaction was completed, the temperature was lowered to 0-5°C, and added slowly Aqueous sodium hydroxide solution, control the internal temperature to be lower than 20 degrees Celsius, add and stir for 0.5h, filter out the solid with a Buchner funnel, rinse twice with water, and dry to obtain 15.9g of off-white solid with a purity of 98.2% and a yield of 79.6 %.
[0043] Synthesis of Compound (II)
[0044] Pyridine (8.7 g, 0.11 mol) and compound I (20.0 g, 0.1 mol) were successively a...
Embodiment 3
[0050] 3-benzyl-6-bromo-2-methoxyquinoline, including the following four steps:
[0051] Synthesis of Compound (I)
[0052] Phosphorus oxychloride (23.0 g, 0.15 mol) was added dropwise to a solution of N,N-dimethylformamide (11.0 g, 0.15 mol) in acetonitrile (100 ml) at 10 °C, and stirred at 10 to 20 °C after the addition was complete. After the reaction for 0.5h, p-bromoaniline (17.2g, 0.1mol) was added at 10-20°C, the temperature was raised to 80°C for 4-6h after the addition, and the reaction was controlled by HPLC. After the reaction was completed, the temperature was lowered to 0-5°C and slowly added Sodium hydroxide aqueous solution, control the internal temperature to be lower than 20 degrees Celsius, add and stir for 0.5h, filter out the solid with a Buchner funnel, rinse twice with water, and dry to obtain 17.1g of off-white solid with a purity of 98.5% and a yield of 85.4 %.
[0053] Synthesis of Compound (II)
[0054] Triethylamine (10.5g, 0.11mol) and compound I...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com