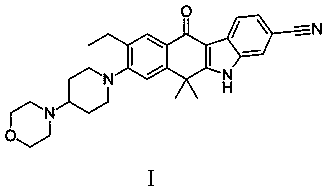
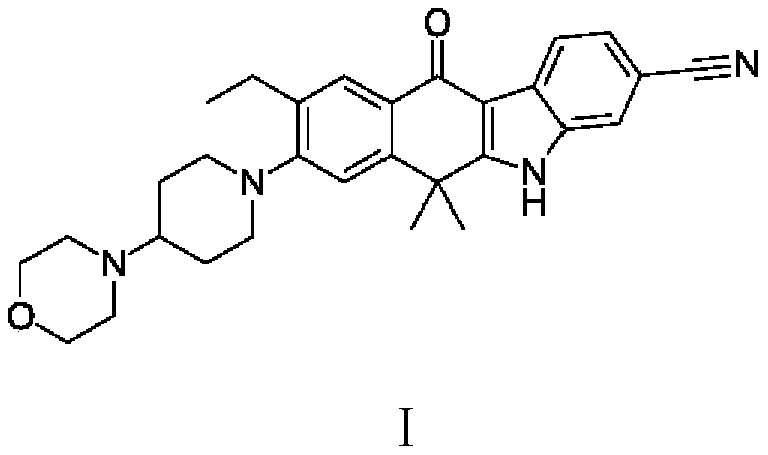
A kind of alectinib intermediate and the preparation method of alectinib
A technology for intermediates and compounds, applied in the field of preparation of alectinib intermediates and alectinib, can solve the problems of high price and unfavorable green industrial production, and achieve high product yield and purity, mild conditions, The effect of a short process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
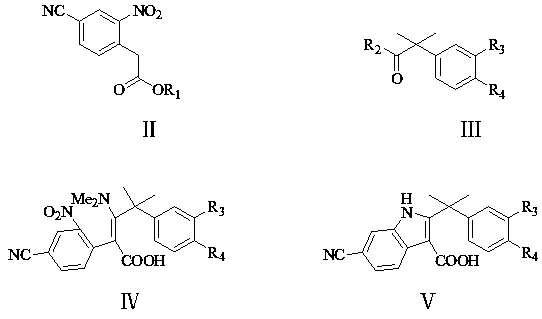
[0075] Example 1: 3-dimethylamino-4-methyl-4-(4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl-2-(2 -Preparation of nitro-4-cyano)phenyl n-pent-2-enoic acid (IV1)
[0076]
[0077] To a 500 ml four-neck flask connected with stirring, a thermometer, a reflux condenser and a dropping funnel, add 180 grams of tetrahydrofuran, 14.6 grams (0.13 moles) of potassium tert-butoxide, between 40 and 45 ° C, dropwise add 22.0 grams of ( 0.1 mol) 2-nitro-4-cyanophenylacetic acid methyl ester (Ⅱ1), 40.0 grams (0.106 mol) 2-methyl-2-(4-ethyl-3-[4-(morpholine-4- Base) piperidin-1-yl] the mixture of phenylpropionyl chloride (Ⅲ1) and 100 grams of tetrahydrofuran, about 3 hours to drop, 45 to 50 ° C stirring reaction for 4 hours. Cool to 30 to 35 ° C, add 16.3 grams of ( 0.2 mole) dimethylamine hydrochloride, stirred and reacted at 30 to 35° C. for 4 hours. Cooled to 20 to 25° C., added 25.0 grams of 20% aqueous sodium hydroxide solution, and stirred and reacted at 20 to 25° C. for 3 hours...
Embodiment 2
[0078] Example 2: 3-dimethylamino-4-methyl-4-(4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl-2-(2 -Preparation of nitro-4-cyano)phenyl n-pent-2-enoic acid (IV1)
[0079]To a 500 ml four-neck flask connected with a stirring, thermometer, reflux condenser and dropping funnel, add 150 g of N,N-dimethylformamide, 15.0 g (0.13 moles) of potassium tert-butoxide, 40 to 45°C Between, drop 22.0 grams (0.1 mol) methyl 2-nitro-4-cyanophenylacetate (Ⅱ1), 37.5 grams (0.1 mol) 2-methyl-2-(4-ethyl-3-[ A mixture of 4-(morpholin-4-yl)piperidin-1-yl]phenylpropionic acid methyl ester (Ⅲ2) and 100 grams of N,N-dimethylformamide, about 3 hours, dropwise, 50 Stir and react at 55°C for 3 hours. Cool to 30 to 35°C, add 16.3 grams (0.2 moles) of dimethylamine hydrochloride, stir and react at 30 to 35°C for 4 hours. Cool to 20 to 25°C, add 50.0 grams of 30% Potassium carbonate aqueous solution, stirred and reacted at 30 to 35°C for 3 hours. Distilled under reduced pressure to recover N,N-dimethyl...
Embodiment 3
[0080] Example 3: 3-dimethylamino-4-methyl-4-(4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl-2-(2 -Preparation of nitro-4-cyano)phenyl n-pent-2-enoic acid (IV1)
[0081] Add 150 g of N,N-dimethylformamide and 8.8 g (0.13 moles) of sodium ethylate to a 500 ml four-necked flask connected with a stirring, thermometer, reflux condenser and dropping funnel, between 60 and 65°C , add dropwise 23.5 grams (0.1 moles) of ethyl 2-nitro-4-cyanophenylacetate (Ⅱ2), 39.0 grams (0.1 moles) of 2-methyl-2-(4-ethyl-3-[4- (Morpholine-4-yl) piperidin-1-yl] the mixture of ethyl phenyl propionate (Ⅲ3) and 100 grams of N,N-dimethylformamide, about 3 hours, dropwise, 60 to 65 ℃ stirring reaction for 3 hours. Cooling to 30 to 35 ℃, adding 50.0 grams of 30% potassium carbonate aqueous solution, 30 to 35 ℃ stirring reaction for 3 hours. Cooling to 30 to 35 ℃, adding 16.3 grams (0.2 moles) of dimethylamine hydrochloric acid Salt, stirred and reacted at 40 to 45°C for 3 hours. Distilled under reduce...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chromatographic purity | aaaaa | aaaaa |
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com