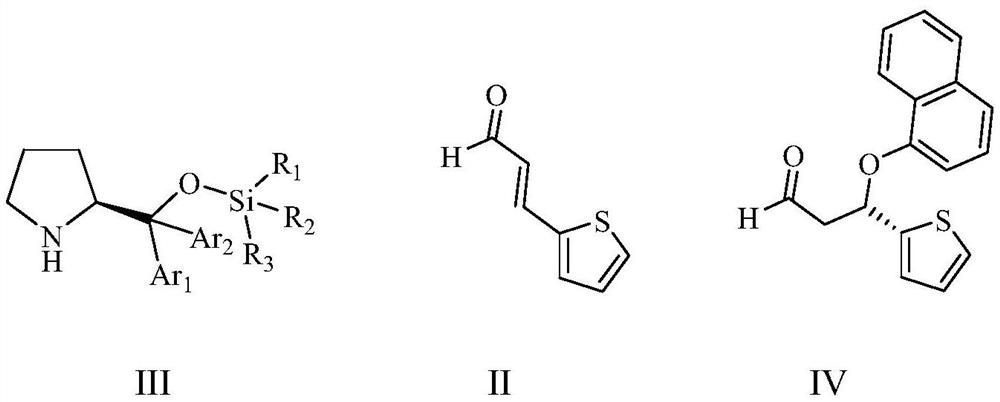
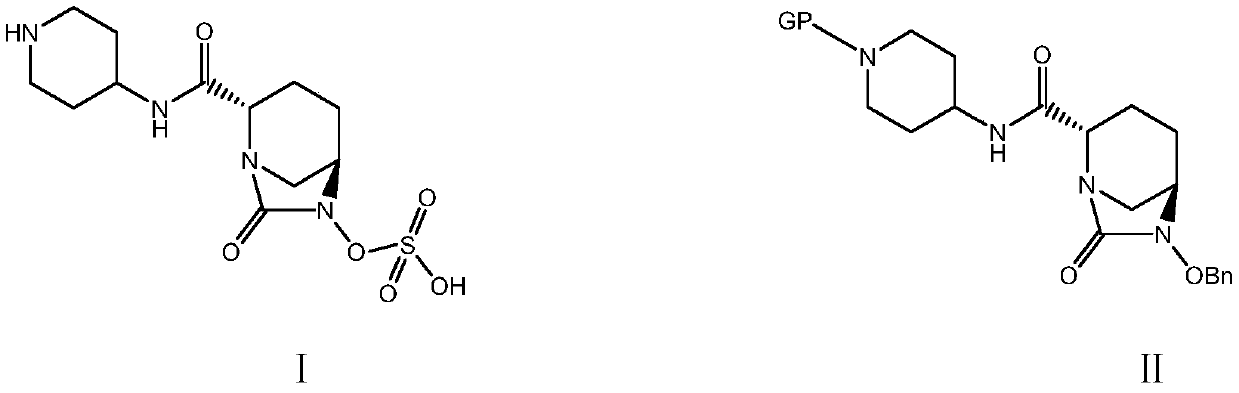
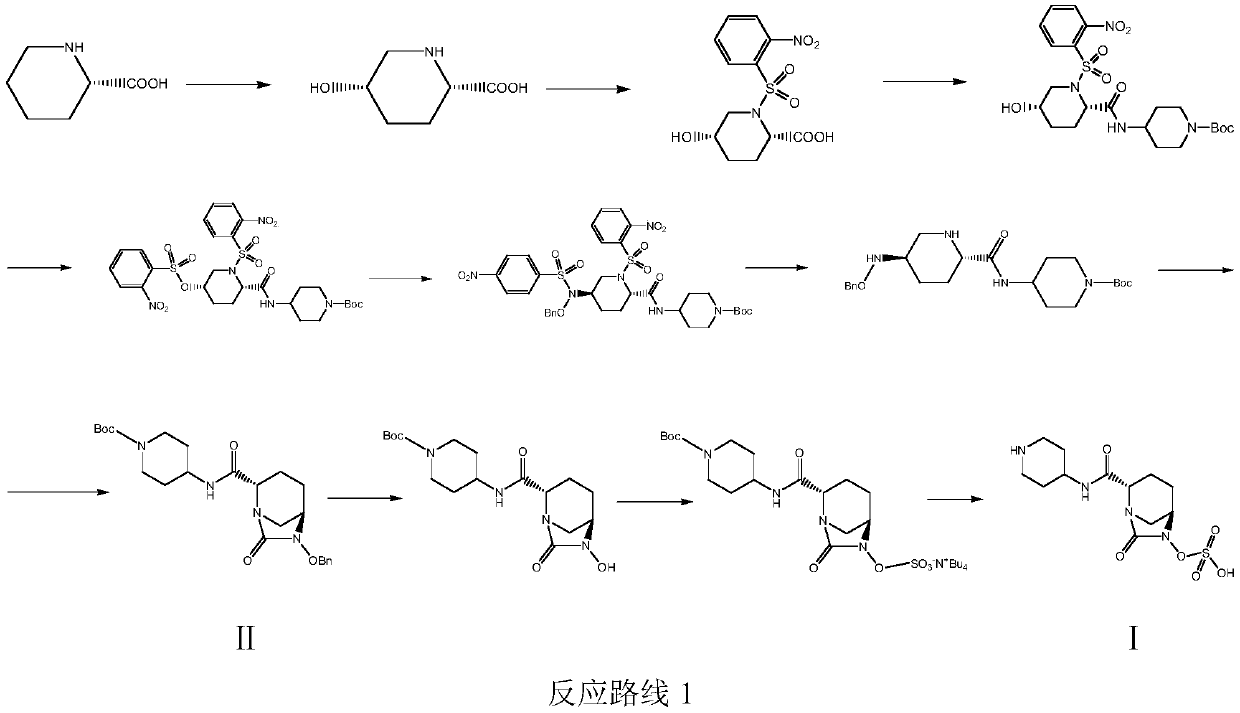
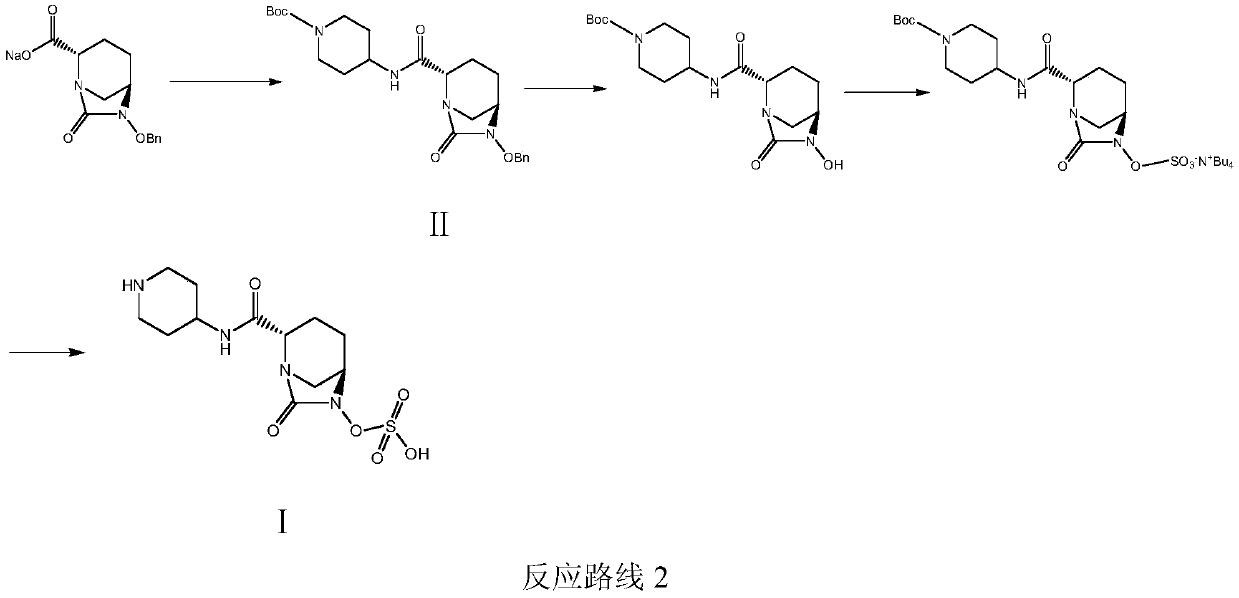
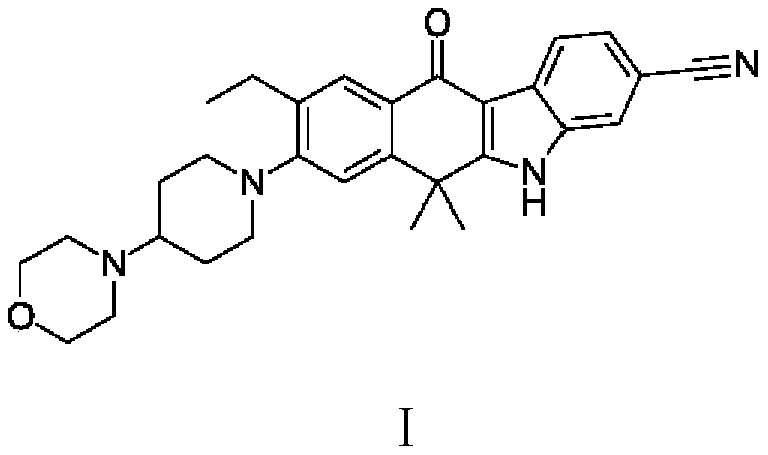
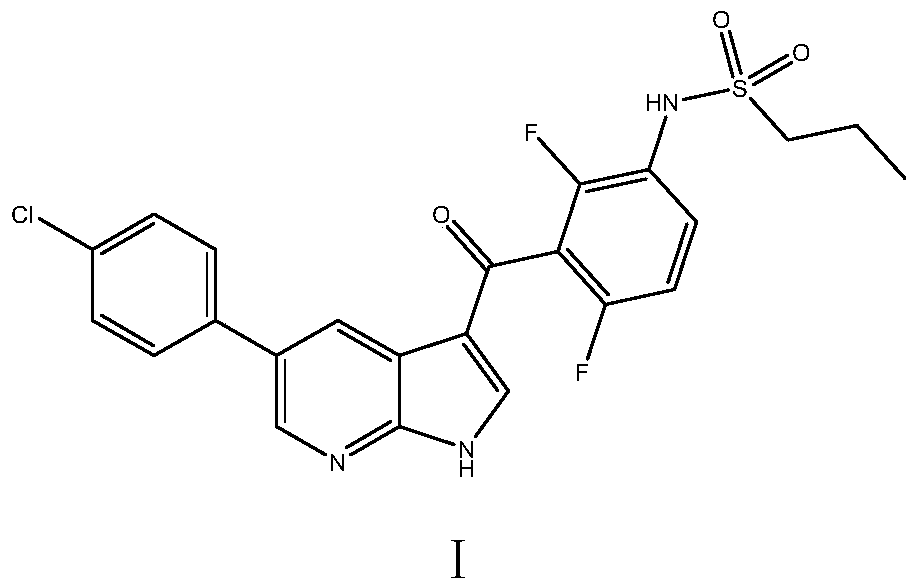
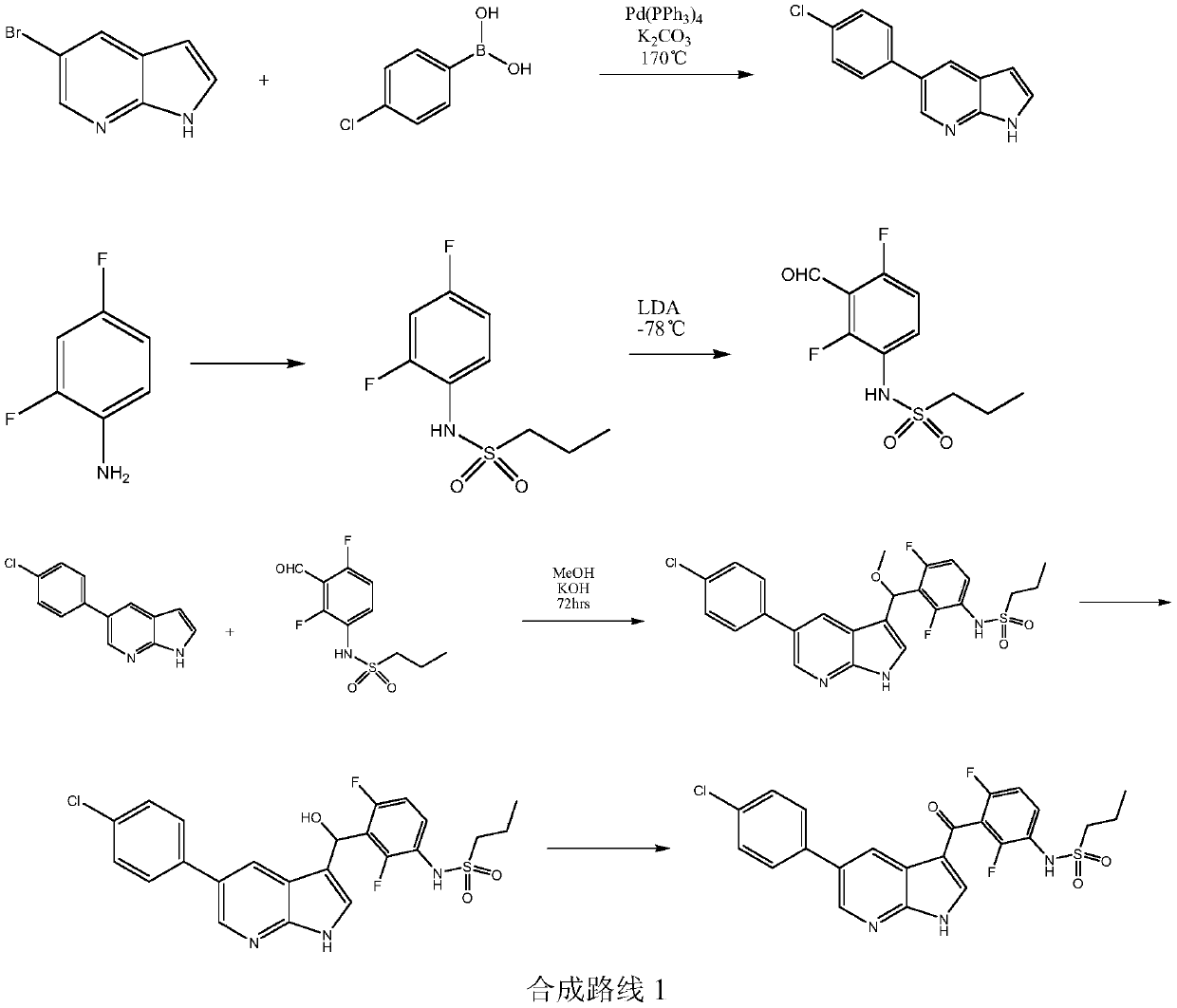
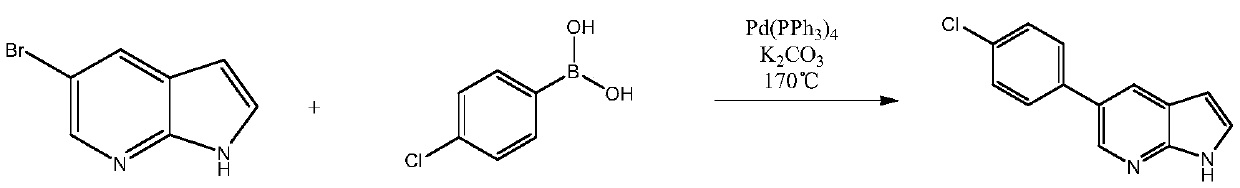
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30results about How to "Conducive to green industrial production" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
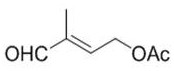
Preparation method of vitamin A acetate intermediate C15 and vitamin A acetate
ActiveCN111484524AHigh reactivityHigh purityOrganic compound preparationGroup 5/15 element organic compoundsPhosphorous acidAcetoxy group
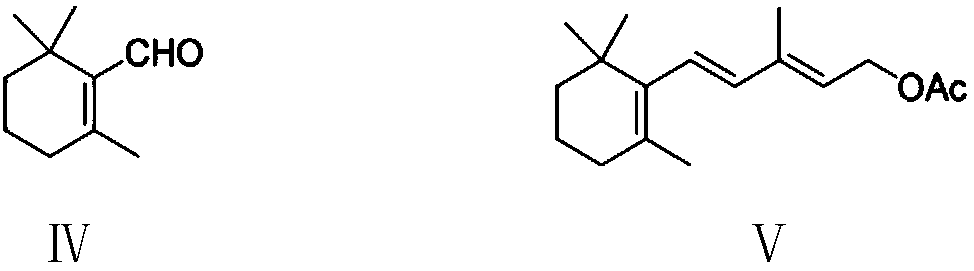
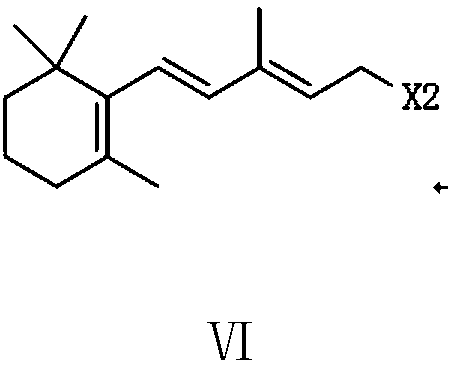
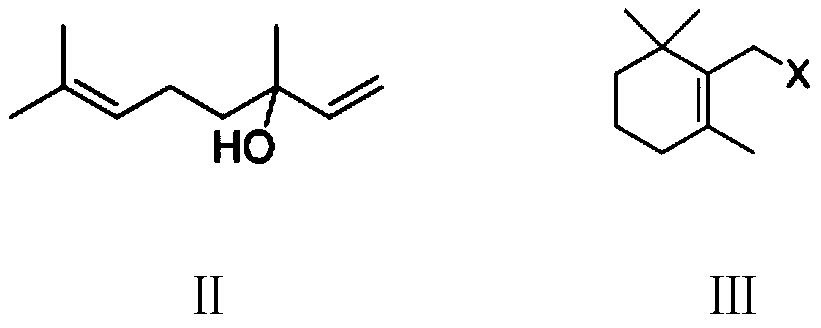
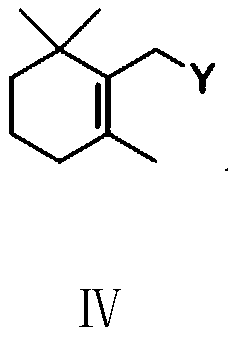
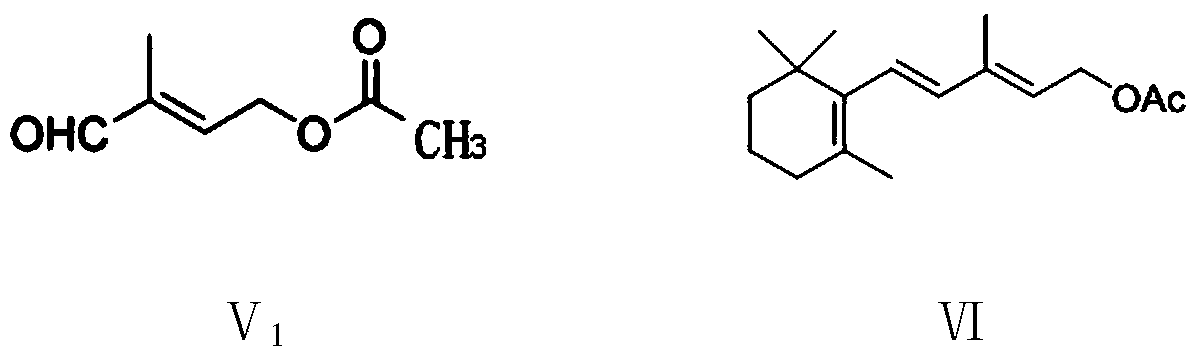
The invention provides a preparation method of a vitamin A acetate intermediate C15 and vitamin A acetate. The method comprises the following steps: taking 1-halogenated-2-methyl-4-acetoxy-2-butene asa raw material, preparing a corresponding Wittig reagent through a substitution reaction with triphenylphosphine or triester phosphite, then carrying out a Wittig reaction with beta-cyclocitral, hydrolyzing an ester group under an alkaline condition, acidifying to obtain a corresponding halide, and carrying out a substitution reaction with triphenylphosphine or triester phosphite again to prepareC15. The vitamin A acetate can be prepared by carrying out a Wittig reaction on the obtained C15 and 2-methyl-4-acetoxy-2-butenal under an alkaline condition. The method has the advantages of singlereaction type, easy operation and realization of reaction conditions, safe and environment-friendly operation, simple post-treatment and low cost; and the reaction activity is strong, the reaction selectivity is high, the atom economy is high, and the target product yield and purity are high.
Owner:XINFA PHARMA
Preparation method of vitamin A ester intermediate C15 and vitamin A ester
ActiveCN111484525AHigh purityHigh yieldGroup 5/15 element organic compoundsHalogenated hydrocarbon preparationPhosphorous acidHalogenation
The invention provides a preparation method of a vitamin A ester intermediate C15 and vitamin A ester. The method comprises the following steps: carrying out a halogenation reaction and a cyclizationreaction on 3, 7-dimethyl-3-hydroxy-1, 6-octadiene as an initial raw material, carrying out a substitution reaction on the obtained product and triphenylphosphine or triester phosphite to prepare a corresponding Wittig reagent, carrying out a Wittig reaction on the Wittig reagent and 2-methyl-4-acetoxy-2-butenal, performing acidifying, hydrolyzing and acidifying the obtained product, and carryingout a substitution reaction on the hydrolyzed and acidified product and triphenylphosphine or triester phosphite to prepare C15. The vitamin A ester can be prepared by carrying out a Wittig reaction on the obtained C15 and 2-methyl-4-R3 substituent carbonyloxy-2-butenal. The method has the advantages of single reaction type, easy operation and realization of reaction conditions, safe and environment-friendly operation, simple post-treatment and low cost; and the reaction activity is strong, the reaction selectivity is high, the atom economy is high, and the target product yield and purity arehigh.
Owner:XINFA PHARMA
3-aminomethyl tetrahydrofuran preparation method
ActiveCN110627752AReduce productionConducive to green industrial productionOrganic chemistryWastewaterNitromethane
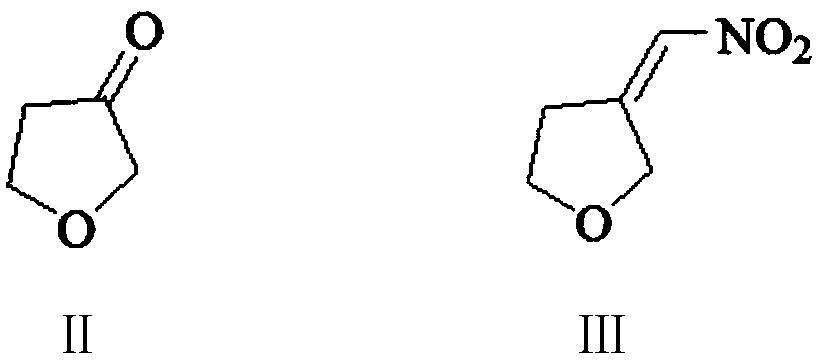
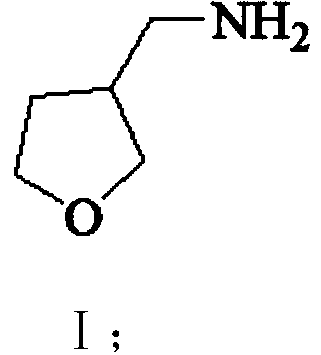
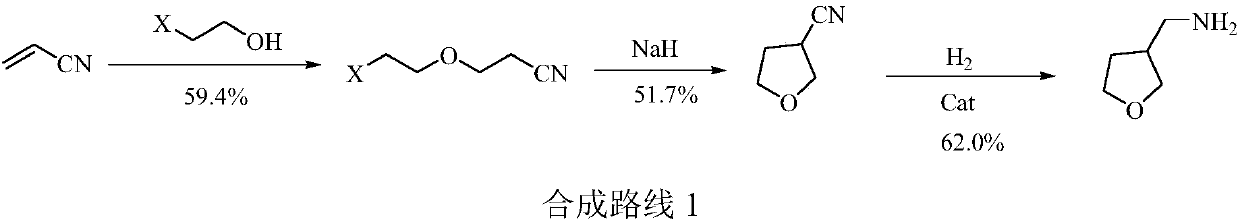
The invention provides a 3-aminomethyl tetrahydrofuran preparation method, which comprises: carrying out a condensation reaction by using tetrahydrofuran-3-one and nitromethane as raw materials to obtain 3-nitromethylene tetrahydrofuran, and carrying out catalytic hydrogenation reduction to obtain 3-aminomethyl tetrahydrofuran. According to the invention, the method has advantages of cheap and easily available raw materials, good stability, low cost, simple preparation steps, safe and simple operation, easy reaction achieving, little wastewater generation, environmental protection, few side reactions, high reaction selectivity, good atom economy, high product purity and high yield, and is suitable for industrial production.
Owner:XINFA PHARMA
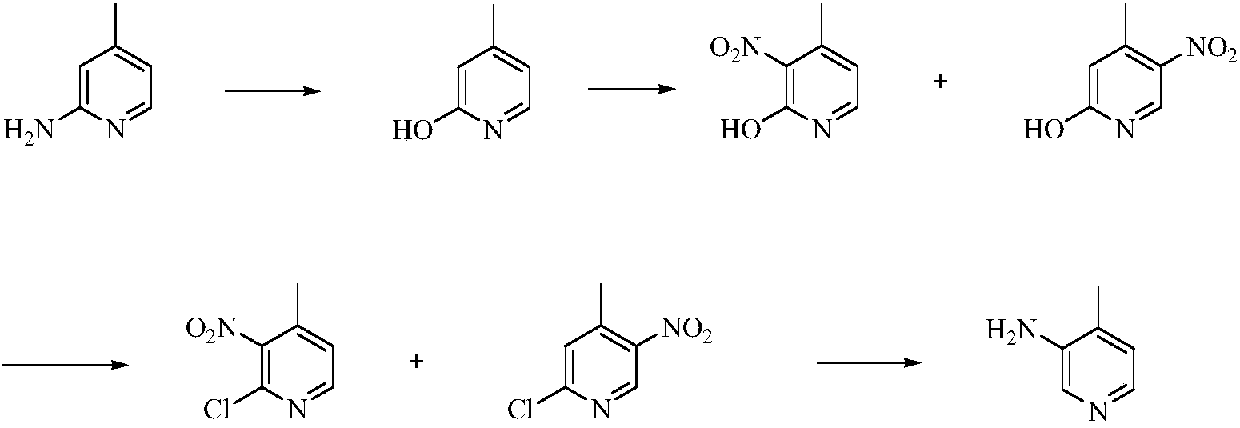
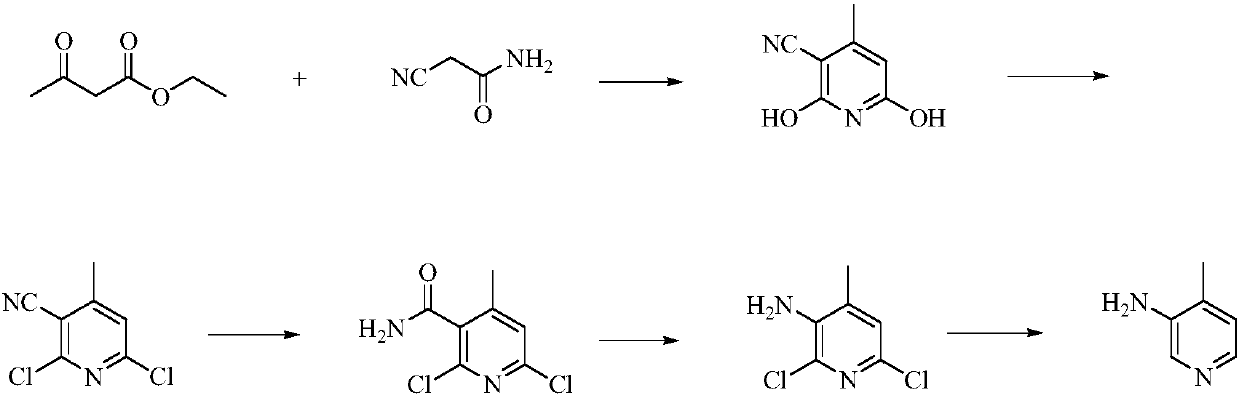
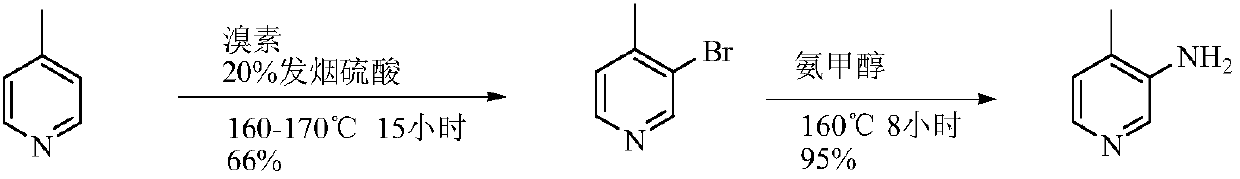
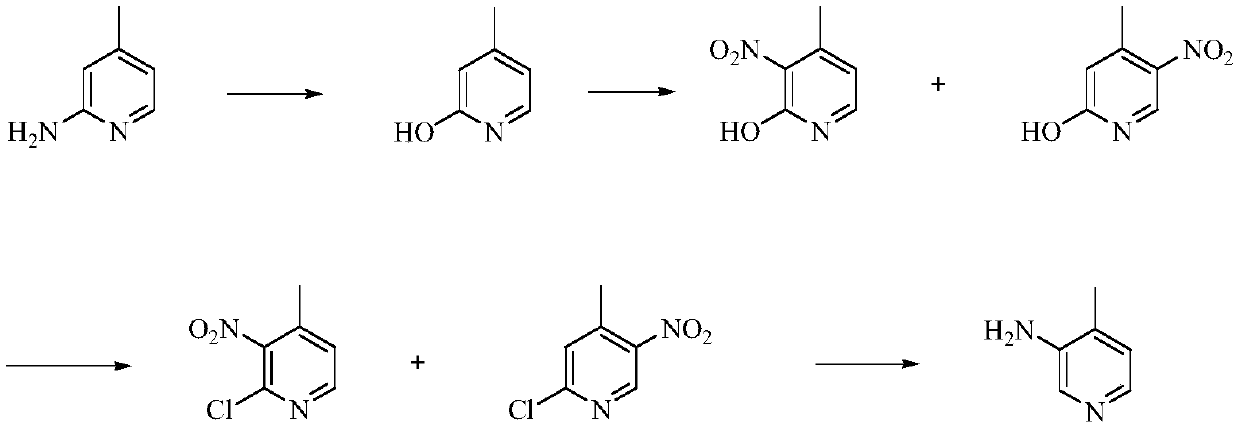
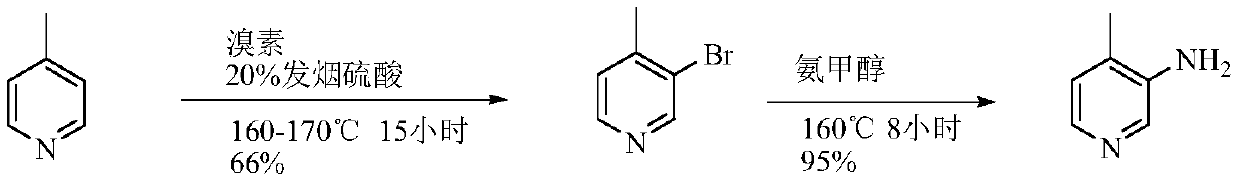
Low-cost simple and convenient preparation method of 3-amino-4-methylpyridine
The invention relates to a low-cost simple and convenient preparation method of 3-amino-4-methylpyridine. The method comprises the following steps: performing addition on halogenated crotonaldehyde asa raw material and nitromethane, performing methylenenation condensation, performing aminopyridine cyclization to obtain 4-methyl-3-nitropyridine, and performing reduction to prepare the 3-amino-4-methylpyridine. According to the preparation method provided by the invention, the raw materials used in the method are cheap and easy to obtain, the conditions are mild, the operation is simple, convenient and safe, the reaction selectivity is high, the product yield and purity are high, and the costs are low; and in the process, the atomic economy is high, and three waste (waste water, waste gas and solid waste) is less.
Owner:XINFA PHARMA
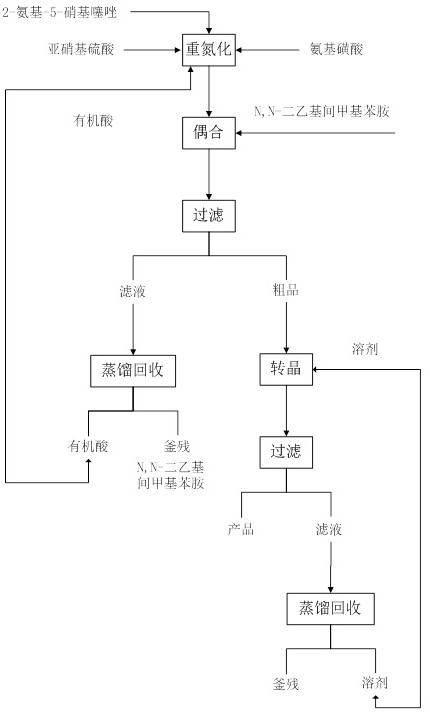
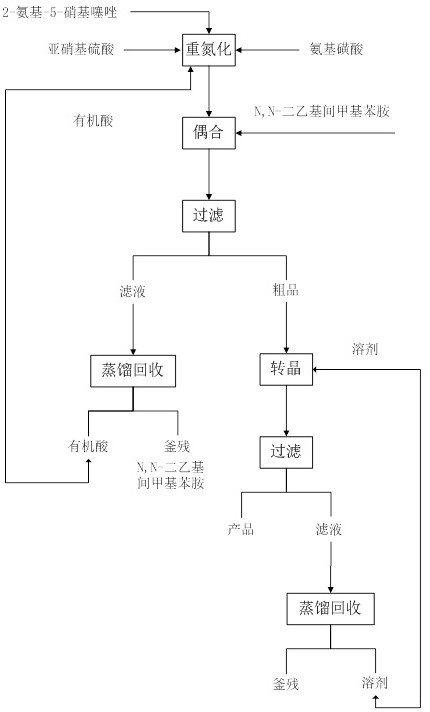
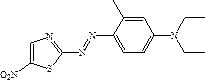
Solvent-free coupling synthesis process of disperse blue 360
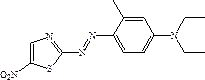
The invention discloses a solvent-free coupling synthesis process of disperse blue 360. The process comprises the following steps: adding 2-amino-5-nitrothiazole into an organic acid medium, controlling the temperature, dropwise adding nitroso-sulfuric acid and carrying out a diazotization reaction to obtain a diazonium solution; adding N, N-diethyl m-methylaniline into a coupling reactor to carryout solventless reaction, cooling to -10 to 10 DEG C, adding the diazonium solution to carry out coupling reaction until the coupling is finished, filtering and washing to obtain a crude product; andobtaining a high-purity fine blue crystal, namely the disperse blue 360 through a crystal transformation process. According to the solvent-free coupling synthesis process of disperse blue 360, pure disperse blue 360 crystals can be obtained, the yield is high, the produced organic acid and crystal transformation solvent can be completely recycled, energy is saved, waste is reduced, the productioncost is greatly reduced, multiple purposes are achieved, and the process has good practical significance for achieving large-scale clean production.
Owner:JIANGSU HANSYN PHARMA
Simple preparation method of vemurafenib and analogues thereof
ActiveCN109970733AConducive to green industrial productionReduce productionOrganic chemistryEnvironmental resistanceN dimethylformamide
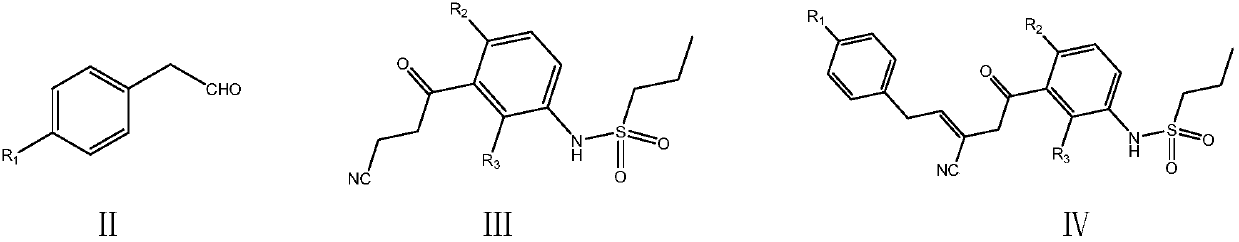
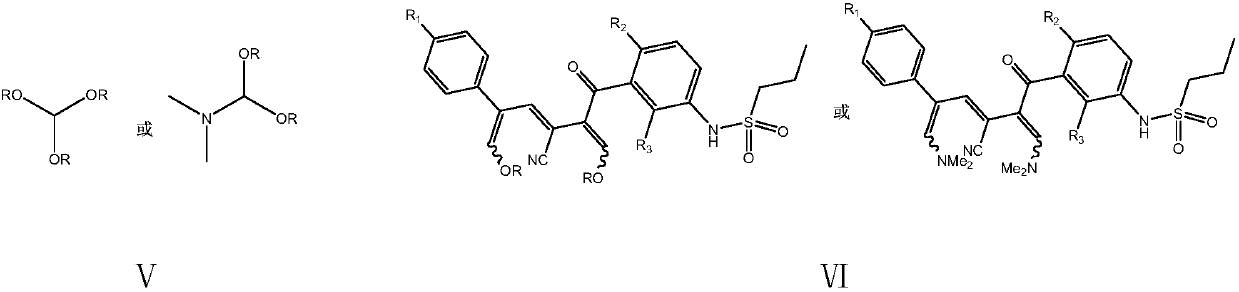
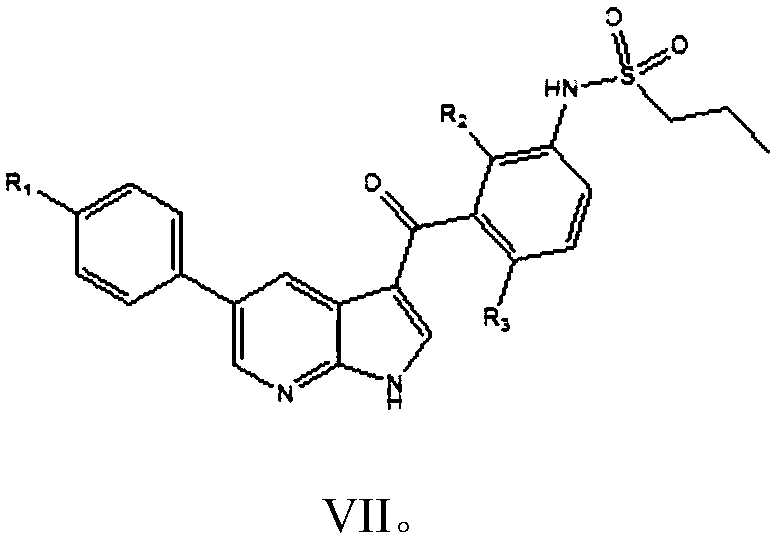
The invention provides a simple preparation method of vemurafenib and analogues thereof. The method includes: subjecting para-substituted phenylacetaldehyde II and N-[2, 4-disubstituted-3-(cyanopropionyl)phenyl]n-propanesulfonamide III to azeotropic dewatering and condensation reaction under alkaili catalysis, condensing the obtained condensation product and the methylenation reagent V(N, N-dimethylformamide or tri-orthoformate), and then performing cyclization with ammonia to obtain vemurafenib or analogues thereof. The method for preparation of vemurafenib provided by the invention has the characteristics of low cost, mild process conditions, low operation requirements, short reaction time, high production efficiency, simple process operation, little waste water production, green and environmental protection, high yield and purity, and is beneficial to green industrial production of vemurafenib. At the same time, the method provided by the invention can prepare vemurafenib analogues,and is of important significance for the drug efficacy study of similar compounds.
Owner:XINFA PHARMA
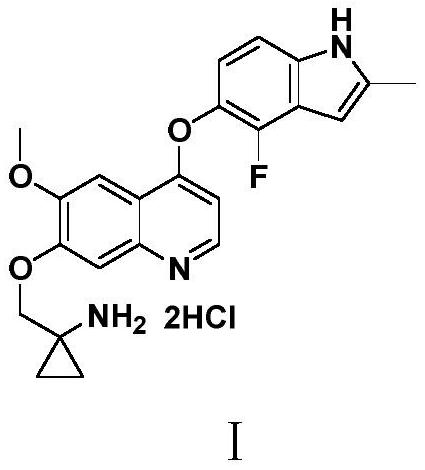
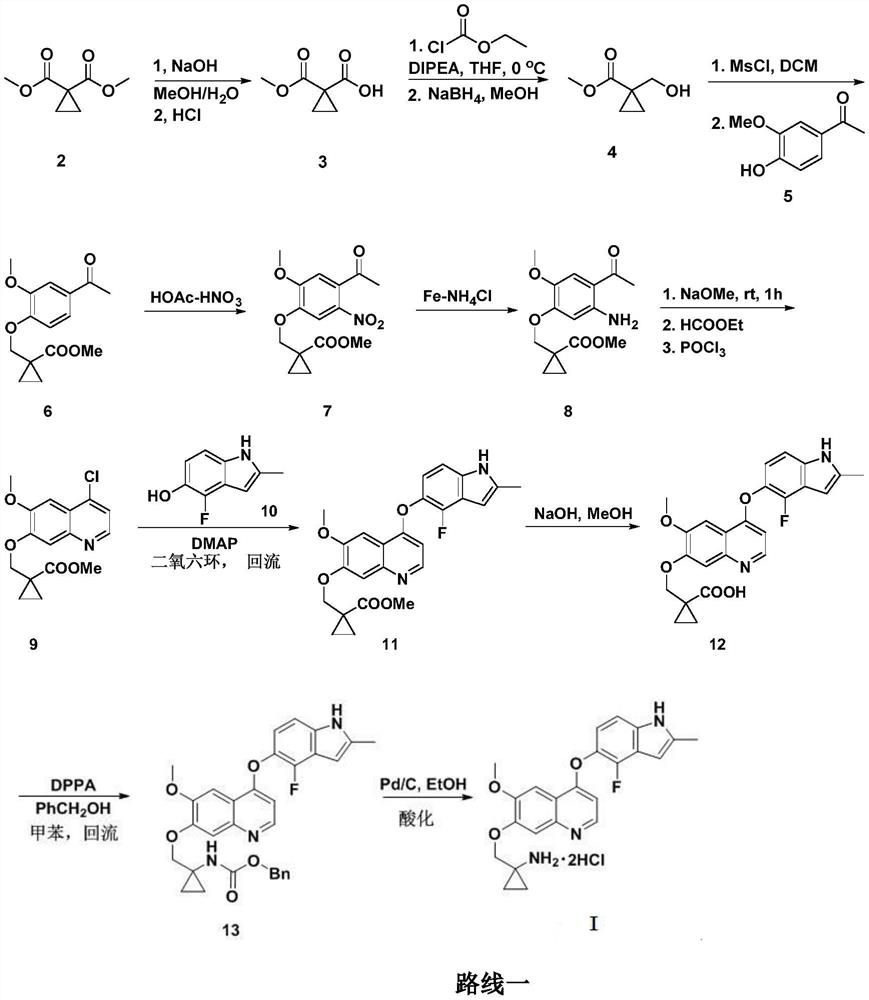
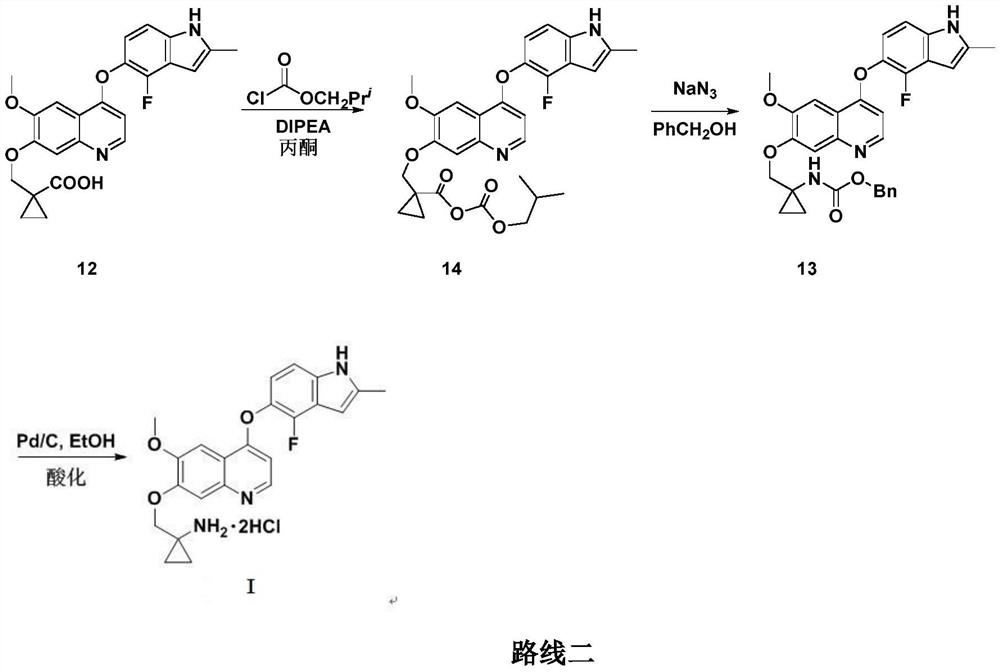
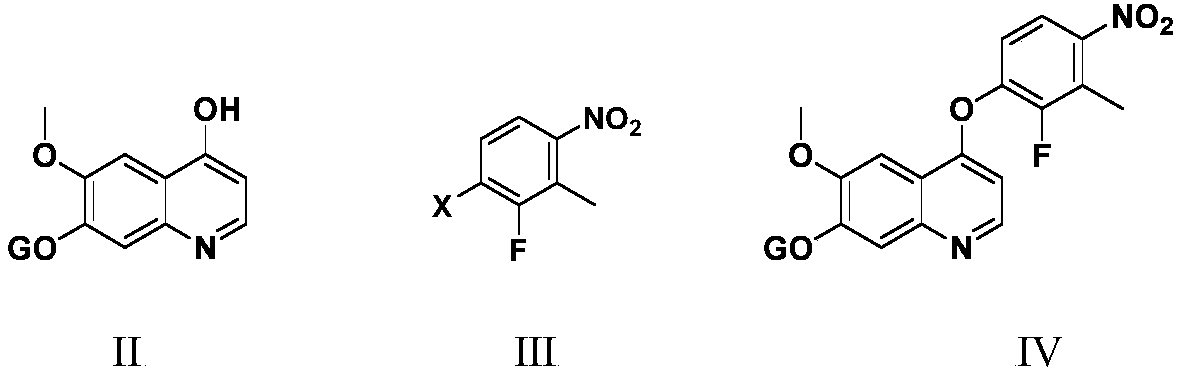
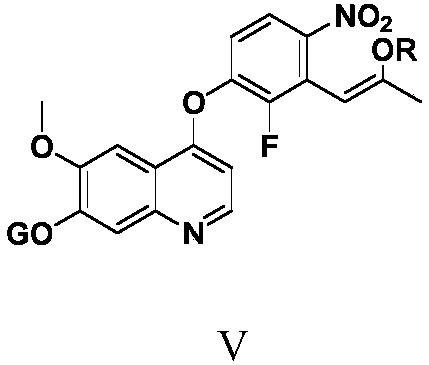
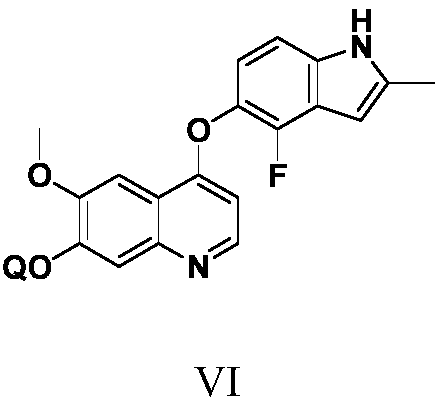
Anlotinib hydrochloride intermediate and preparation method of Anlotinib hydrochloride
The invention provides an anlotinib hydrochloride intermediate and a preparation method of anlotinib hydrochloride. 4-hydroxy-6-methoxyl-7-GO substituted quinolone and 2-fluorine-3-halogenate-6-bitrotoluene are used for generating 4-(2-fluorine-3-methyl-4- nitrobenzophenone)epoxide-6-methoxyl-7-GO substituted quinolone under action of an acid-binding agent through substitution reaction, and then condensation reaction is conducted with triglyceride orthoacetate to obtain 4-[2-fluorine-3-(2-alkyloxyethyl-propylene-1-base)-4-nitrobenzophenone]epoxide-6-methoxyl-7-GO substituted quinolone, and then under existing of a catalyst, reduction-cyclization reaction is conducted to obtain the anlotinib hydrochloride intermediate. The obtained anlotinib hydrochloride intermediate can be used for preparing the anlotinib hydrochloride according to the prior art or the preparation method of the anlotinib hydrochloride. According to the anlotinib hydrochloride intermediate and the preparation method ofthe anlotinib hydrochloride, raw materials are cheap and easy to obtain, the technology process is simple and short, operation is easy and convenient, safety and environmental protection are achieved, the cost is low, and selectivity, the yield coefficient and the purity are high.
Owner:XINFA PHARMA
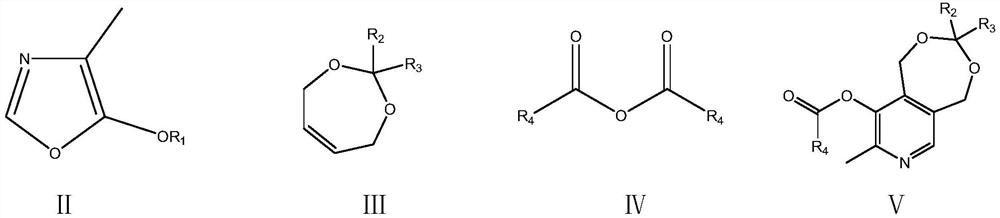
A kind of preparation method of vitamin B6
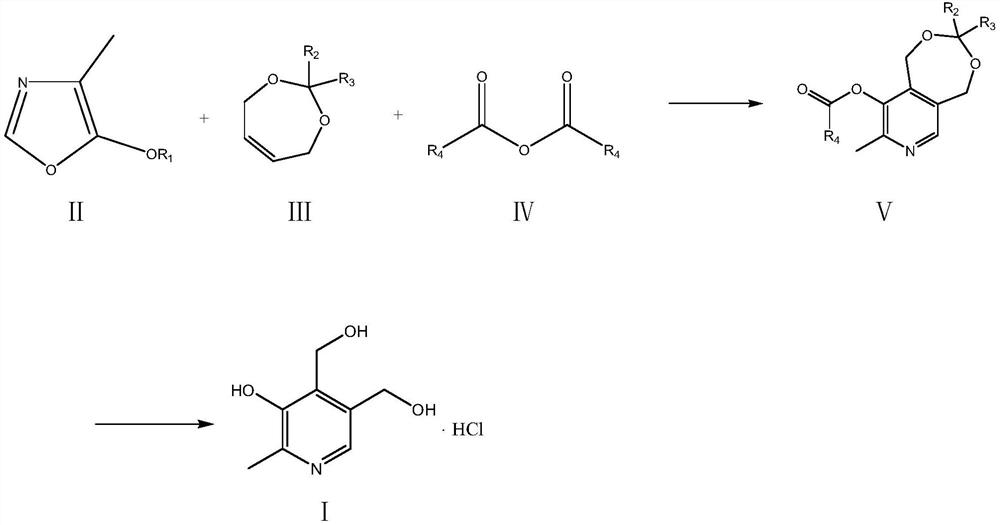
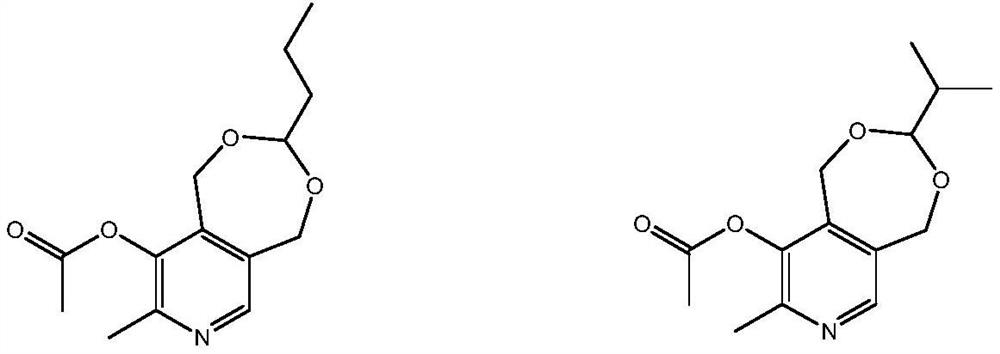
The invention relates to a preparation method of a high content vitamin B6. According to the method, 1,5-dihydro-3,3-disubstituent group-8-methyl-9-alkyl carbonyl oxypyrido [3,4-e]-1,3-dioxane is prepared by the Diels-Alder addition reaction, aromatization reaction and esterification reaction of 4-methyl-5-alkoxyl-oxazole and 2,2-disubstituent group-4,7-dihydro-1,3-dioxepine in the presence of ananhydride through the "one-pot method", and then vitamin B6 is prepared by deprotection. According to the method, the stability of the raw materials of 4-methyl-5-alkoxyl-oxazole and 2,2-disubstituentgroup-4,7-dihydro-1,3-dioxepine are ensured, the reaction is thorough, and the selectivity is high, so that the method provides guarantees for the preparation of high content medicinal vitamin B6.
Owner:XINFA PHARMA
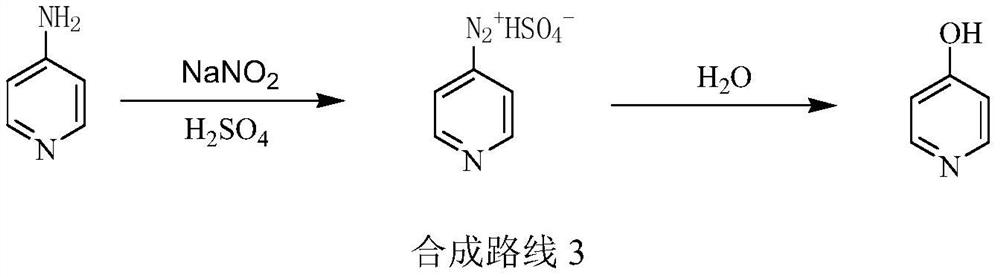
A kind of preparation method of 3-amino-4-picoline
The invention relates to a low-cost simple and convenient preparation method of 3-amino-4-methylpyridine. The method comprises the following steps: performing addition on halogenated crotonaldehyde asa raw material and nitromethane, performing methylenenation condensation, performing aminopyridine cyclization to obtain 4-methyl-3-nitropyridine, and performing reduction to prepare the 3-amino-4-methylpyridine. According to the preparation method provided by the invention, the raw materials used in the method are cheap and easy to obtain, the conditions are mild, the operation is simple, convenient and safe, the reaction selectivity is high, the product yield and purity are high, and the costs are low; and in the process, the atomic economy is high, and three waste (waste water, waste gas and solid waste) is less.
Owner:XINFA PHARMA
Preparation method of pyridine derivative
ActiveCN110483383ARaw materials are cheap and easy to getShort routeOrganic chemistryWastewaterKetone
The invention relates to a preparation method of pyridine derivatives, which comprises the following steps: by using piperidine-4-ketone hydrochloride as a raw material, carrying out halogenation reaction and elimination reaction to obtain a series of pyridine derivatives. Piperidine-4-ketone hydrochloride and a specific amount of a halogenation reagent are subjected to a halogenation reaction torespectively prepare 3,5-dihalogenated piperidine-4-one, 3,3,5-trihalogenated piperidine-4-one, or 3,3,5,5-tetrahalogenated piperidine-4-one; then performing elimination reaction on the raw materialsand different types of alkaline reagents to obtain pyridine derivatives of which the 4-positions are hydroxyl, amino or dimethylamino groups respectively. The method is simple and convenient to operate, mild in condition, short in technological process, low in wastewater amount, environmentally friendly and low in cost, and green industrial production of the pyridine derivative is facilitated.
Owner:XINFA PHARMA
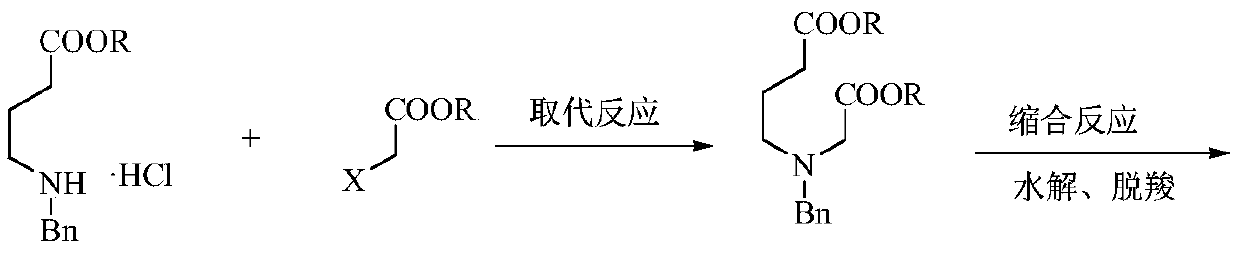
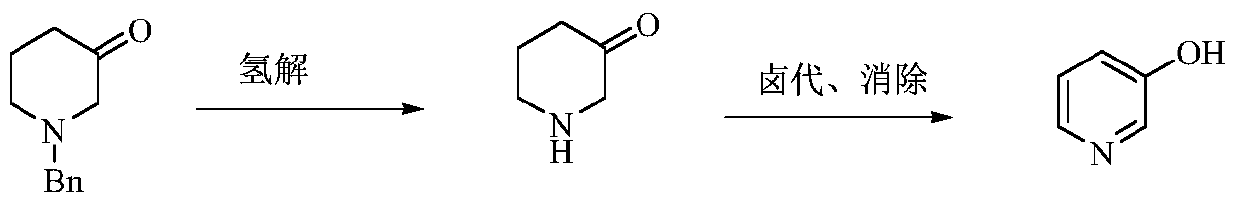
Preparation method of low-cost 3-hydroxypyridine
ActiveCN110240561AFix security issuesSolve environmental problemsOrganic chemistryOperation safetyElimination reaction
The invention relates to a preparation method of low-cost 3-hydroxypyridine. The method comprises the steps of enabling 4-benzylaminobutyrate hydrochloride and 2-haloacetate to be subjected to a substitution reaction so as to obtain N-benzyl-3-aza-1, 7-pimelate diester; then, enabling the N-benzyl-3-aza-1, 7-pimelate diester to be subjected to an intramolecular condensation reaction, and carrying out hydrolytic decarboxylation to obtain N-benzylpiperidin-3-one; then, carrying out catalytic hydrogenolysis to remove benzyl so as to obtain piperidin-3-one; enabling the piperidin-3-one and halogen to be subjected to a halogenating reaction so as to obtain 2, 4-dihalogenated piperidine-3-one; then, enabling the 2, 4-dihalogenated piperidine-3-one and an acid-binding agent to be subjected to an elimination reaction to obtain 3-hydroxypyridine. The raw materials used in the preparation method are low in price and easy to obtain, the operation conditions are mild, simple and convenient, and less wastewater is produced; the method is high in operation safety, environmental protection property, product yield and purity, and low in cost.
Owner:XINFA PHARMA
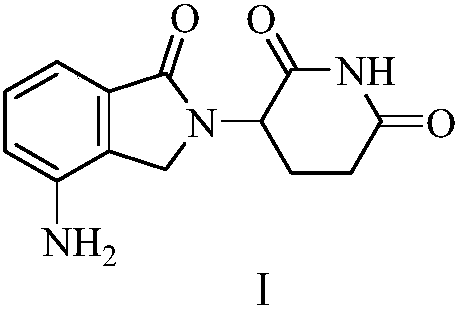
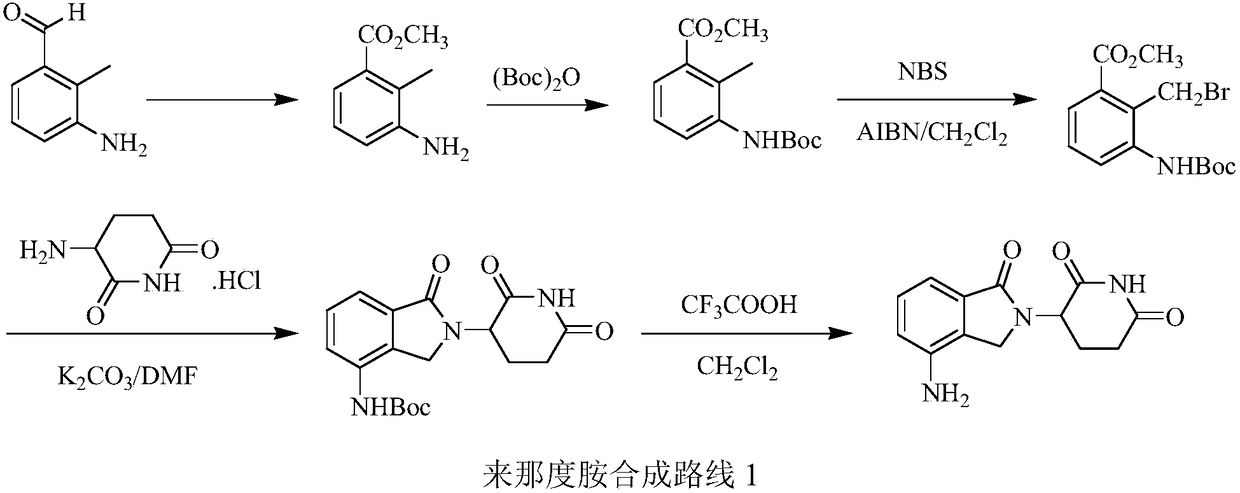
Green production method of low-cost lenalidomide
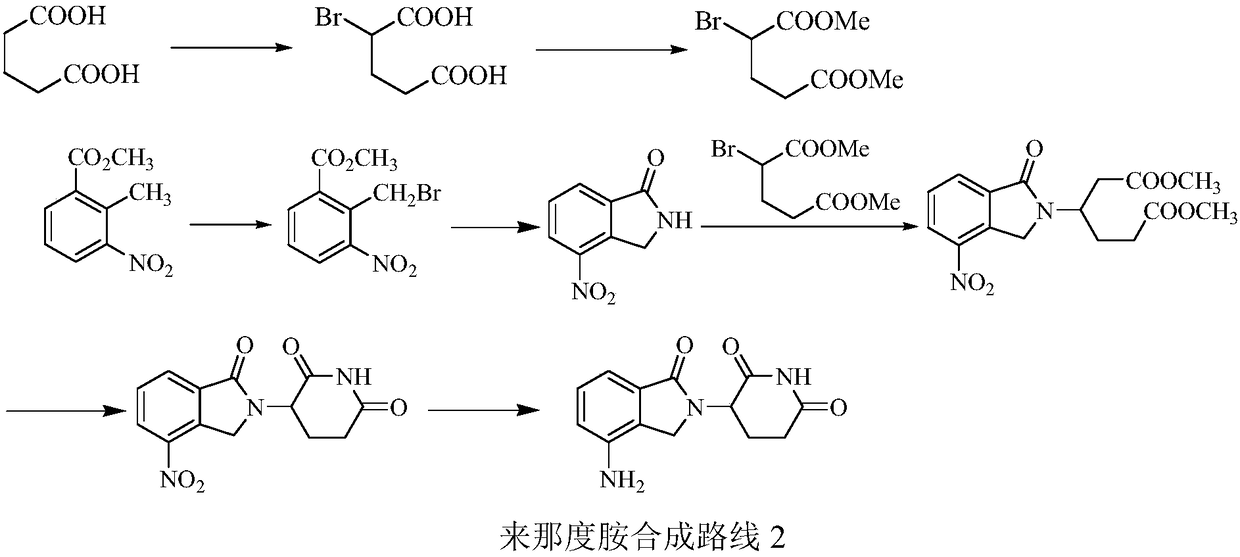
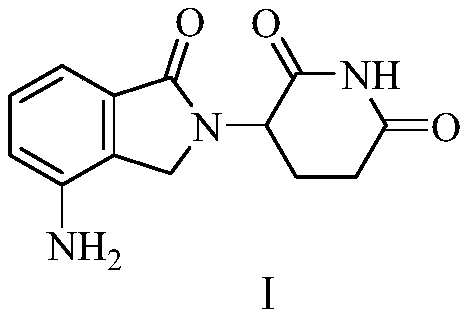
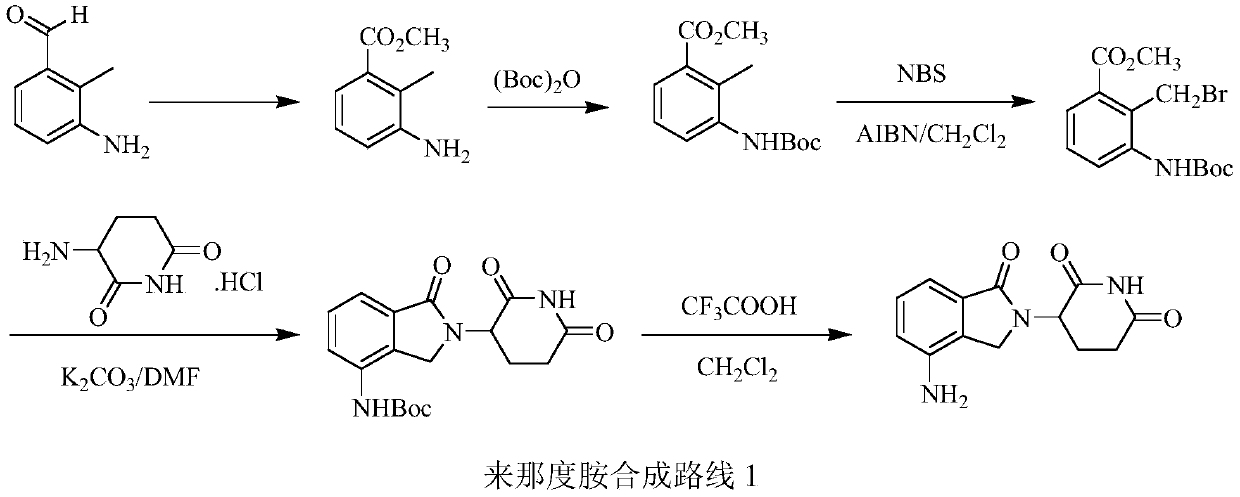
The invention relates to a green production method of low-cost lenalidomide. According to the method, in presence of a solvent and an alkali, 3-aminopiperidine-2,6-dione and 1-halo-acetoacetate are subjected to dehydrogenation halogen acid condensation, dealcoholizing amidation, 2-halo-4-nitrobutanal dehydration and dehydrochlorination or dehydrobromination, 3-(7-nitro-3-oxo-1H-isoindol-2-yl)piperidine-2,6-dione is obtained, the process is completed with a one-pot method, nitro is reduced into amino by catalytic hydrogenation of 3-(7-nitro-3-oxo-1H-isoindol-2-yl)piperidine-2,6-dione, and lenalidomide is prepared. The method has the advantages of cheap and easily available raw materials, short technological process, simple operation and environmental protection and is a production method beneficial to industrialization.
Owner:XINFA PHARMA
A kind of production method of lenalidomide
The invention relates to a green production method of low-cost lenalidomide. According to the method, in presence of a solvent and an alkali, 3-aminopiperidine-2,6-dione and 1-halo-acetoacetate are subjected to dehydrogenation halogen acid condensation, dealcoholizing amidation, 2-halo-4-nitrobutanal dehydration and dehydrochlorination or dehydrobromination, 3-(7-nitro-3-oxo-1H-isoindol-2-yl)piperidine-2,6-dione is obtained, the process is completed with a one-pot method, nitro is reduced into amino by catalytic hydrogenation of 3-(7-nitro-3-oxo-1H-isoindol-2-yl)piperidine-2,6-dione, and lenalidomide is prepared. The method has the advantages of cheap and easily available raw materials, short technological process, simple operation and environmental protection and is a production method beneficial to industrialization.
Owner:XINFA PHARMA
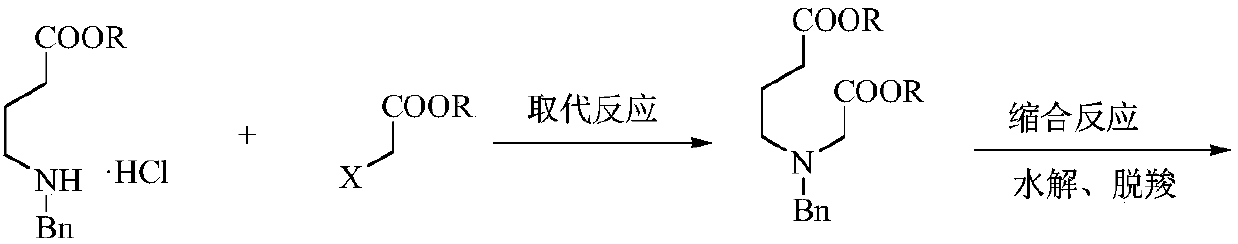
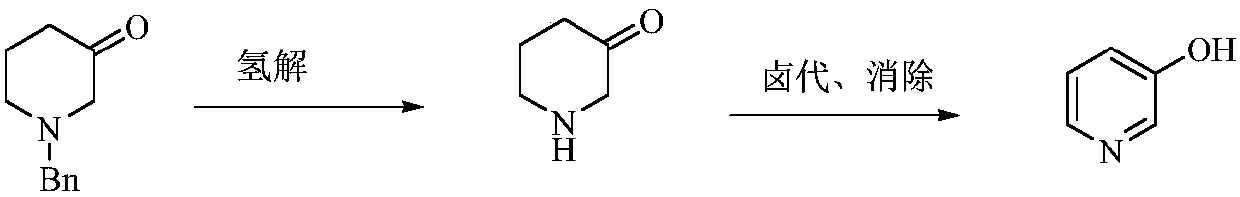
A kind of preparation method of low-cost 3-hydroxypyridine
ActiveCN110240561BFix security issuesSolve environmental problemsOrganic chemistryHydrolysisPyridyne
The invention relates to a preparation method of low-cost 3-hydroxypyridine. The method comprises the steps of enabling 4-benzylaminobutyrate hydrochloride and 2-haloacetate to be subjected to a substitution reaction so as to obtain N-benzyl-3-aza-1, 7-pimelate diester; then, enabling the N-benzyl-3-aza-1, 7-pimelate diester to be subjected to an intramolecular condensation reaction, and carrying out hydrolytic decarboxylation to obtain N-benzylpiperidin-3-one; then, carrying out catalytic hydrogenolysis to remove benzyl so as to obtain piperidin-3-one; enabling the piperidin-3-one and halogen to be subjected to a halogenating reaction so as to obtain 2, 4-dihalogenated piperidine-3-one; then, enabling the 2, 4-dihalogenated piperidine-3-one and an acid-binding agent to be subjected to an elimination reaction to obtain 3-hydroxypyridine. The raw materials used in the preparation method are low in price and easy to obtain, the operation conditions are mild, simple and convenient, and less wastewater is produced; the method is high in operation safety, environmental protection property, product yield and purity, and low in cost.
Owner:XINFA PHARMA
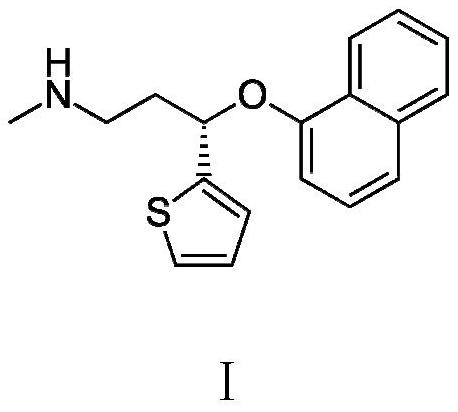
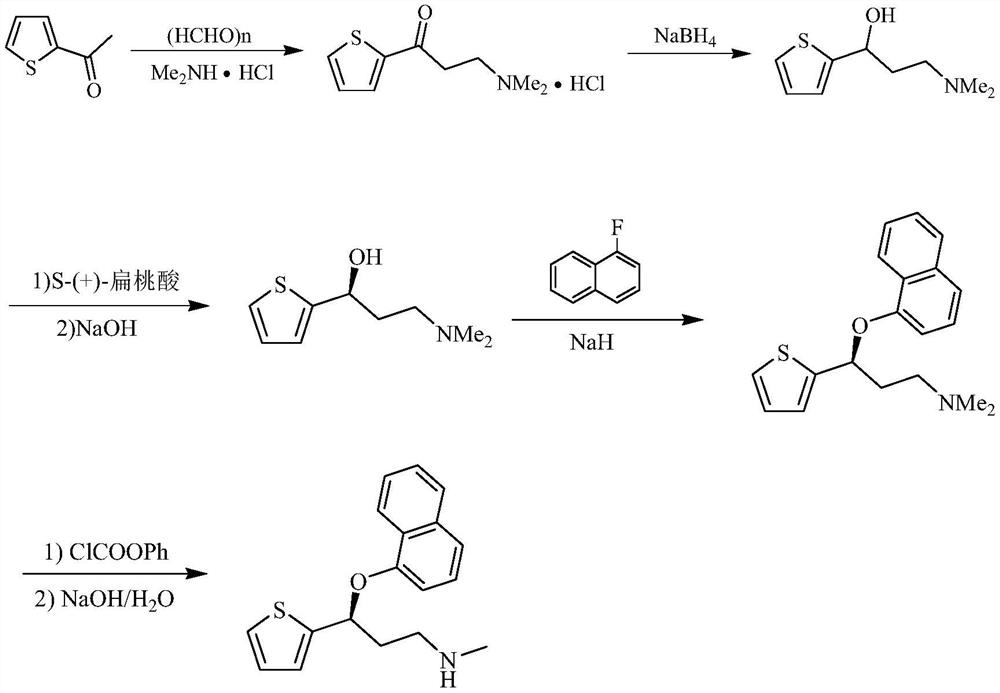
A kind of preparation method of duloxetine
ActiveCN113429380BAvoid racemizationReduce productionOrganic chemistry methodsDuloxetinePtru catalyst
Owner:XINFA PHARMA
A kind of preparation method of 3-aminomethyltetrahydrofuran
ActiveCN110627752BReduce productionConducive to green industrial productionOrganic chemistryWastewaterNitromethane
The invention provides a preparation method of 3-aminomethyltetrahydrofuran, which uses tetrahydrofuran-3-ketone and nitromethane as raw materials to obtain 3-nitromethylene tetrahydrofuran through condensation reaction, and then obtains 3-nitromethylene tetrahydrofuran through catalytic hydrogenation reduction Aminomethyltetrahydrofuran. The method of the invention has cheap and easy-to-obtain raw materials, good stability, and low cost; simple preparation steps, safe and convenient operation, and easy realization of the reaction; less waste water generation, environmental protection; less side reactions, high reaction selectivity, and good atom economy. High purity and yield, suitable for industrial production.
Owner:XINFA PHARMA
A kind of convenient preparation method of raylebactam intermediate
ActiveCN109928970BReduce productionReduce typesOrganic chemistryBulk chemical productionRelebactamPtru catalyst
The invention discloses a simple preparation method of Relebactam intermediate, namely (2S,5R)-N-(1-protective group) piperidine-4-yl-6-benzyloxy-7-oxo-1,6-diazabicyclo[3.2.1]octane - 2- formamide, (2S,5R)-6-benzyloxy-7-oxo-1,6-diazabicyclo[3.2.1]octane-2-carbonyl chloride is prepared from (2S, 5R) -5- benzyloxaminopiperidine -2- formic acid and phosgene, solid phosgene or diphosgene in a solventin the presence of an alkali and a catalyst by epoxidation and acylating chlorination reaction by a one-pot method, and the (2S,5R)-6-benzyloxy-7-oxo-1,6-diazabicyclo[3.2.1]octane-2-carbonyl chlorideis not separated and is directly subjected to amidation reaction with (1-protective group)-4-amino piperidine to obtain the (2S,5R)-N-(1-protective group) piperidine-4-yl-6-benzyloxy-7-oxo-1,6-diazabicyclo[3.2.1]octane - 2- formamide. The method disclosed by the invention is simple in steps, cheap and easily available in raw materials, green and environment-friendly in process, low in cost, high in reaction atom economy, high in purity, yield and selectivity of the obtained intermediate, and beneficial to industrial production.
Owner:XINFA PHARMA
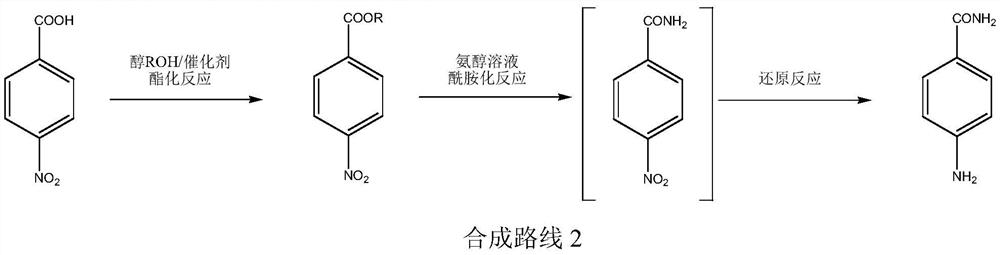
A kind of production technology of p-aminobenzamide
ActiveCN109867604BRaw materials are cheap and easy to getGood reaction selectivityOrganic compound preparationCarboxylic acid amides preparationBenzoic acidPtru catalyst
The invention relates to a production process of p-aminobenzamide. The process utilizes p-nitrobenzoic acid and alcohol to generate p-nitrobenzoic acid ester through catalytic esterification reaction, and then in the presence of hydrogenation catalyst and hydrogen, ammonia Preparation of p-aminobenzamide by amidation reaction in alcohol solution. The raw materials used in the invention are cheap and easy to obtain, easy to operate, less waste water, high in operation safety, high in reaction selectivity, high in product yield and purity, and low in cost.
Owner:XINFA PHARMA
A kind of preparation method of vitamin A acetate intermediate C15 and vitamin A acetate
ActiveCN111484524BHigh reactivityHigh purityOrganic compound preparationGroup 5/15 element organic compoundsPhosphorous acidHydrolysis
Owner:XINFA PHARMA
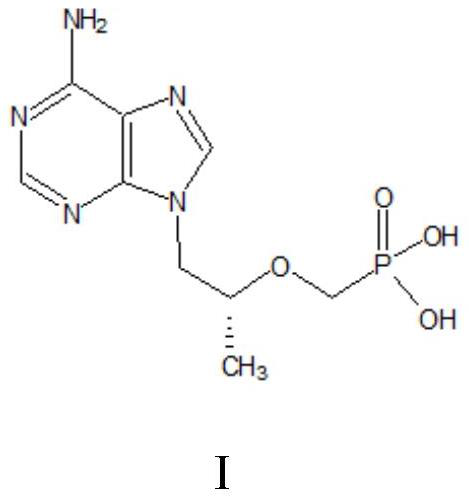
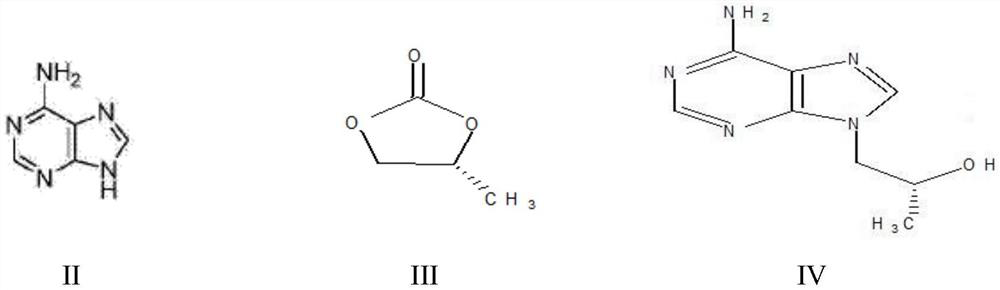
A kind of method utilizing microreactor to prepare tenofovir
ActiveCN110452269BPromote esterolysisReduce productionGroup 5/15 element organic compoundsChemical/physical/physico-chemical microreactorsTosylhydrazoneSide reaction
The invention provides a method for preparing tenofovir using a microreactor. Adopt adenine and (R)-propylene carbonate as raw material to prepare (R)-9-(2-hydroxypropyl) adenine through condensation reaction, then under the effect of magnesium tert-butoxide and p-toluenesulfonyloxyphosphine (R)-9-[2-(diethylphosphonomethoxy)propyl]adenine is prepared by condensation reaction of diethyl ester; then, using a microreactor, hydrogen chloride gas is used as a deesterification reagent to carry out a deesterification reaction Preparation of tenofovir. The deesterification reaction of the present invention adopts hydrogen chloride as a deesterification reagent, and the hydrogen chloride used is cheap and low in cost; adopts quantitative reaction, and utilizes micro-reactor technology to carry out deesterification reaction, improves reaction pressure, temperature, and enhances mixing effect; waste liquid generation Less, green and environmentally friendly; fast reaction rate, high reaction efficiency, less side reactions, high purity and yield of target products, which is conducive to industrial production.
Owner:山东安弘制药有限公司
Anlotinib hydrochloride intermediate and preparation method of anlotinib hydrochloride
The invention provides an anlotinib hydrochloride intermediate and a preparation method of anlotinib hydrochloride. 4-hydroxy-6-methoxyl-7-GO substituted quinolone and 2-fluorine-3-halogenate-6-bitrotoluene are used for generating 4-(2-fluorine-3-methyl-4- nitrobenzophenone)epoxide-6-methoxyl-7-GO substituted quinolone under action of an acid-binding agent through substitution reaction, and then condensation reaction is conducted with triglyceride orthoacetate to obtain 4-[2-fluorine-3-(2-alkyloxyethyl-propylene-1-base)-4-nitrobenzophenone]epoxide-6-methoxyl-7-GO substituted quinolone, and then under existing of a catalyst, reduction-cyclization reaction is conducted to obtain the anlotinib hydrochloride intermediate. The obtained anlotinib hydrochloride intermediate can be used for preparing the anlotinib hydrochloride according to the prior art or the preparation method of the anlotinib hydrochloride. According to the anlotinib hydrochloride intermediate and the preparation method ofthe anlotinib hydrochloride, raw materials are cheap and easy to obtain, the technology process is simple and short, operation is easy and convenient, safety and environmental protection are achieved, the cost is low, and selectivity, the yield coefficient and the purity are high.
Owner:XINFA PHARMA
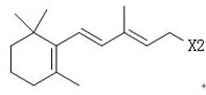
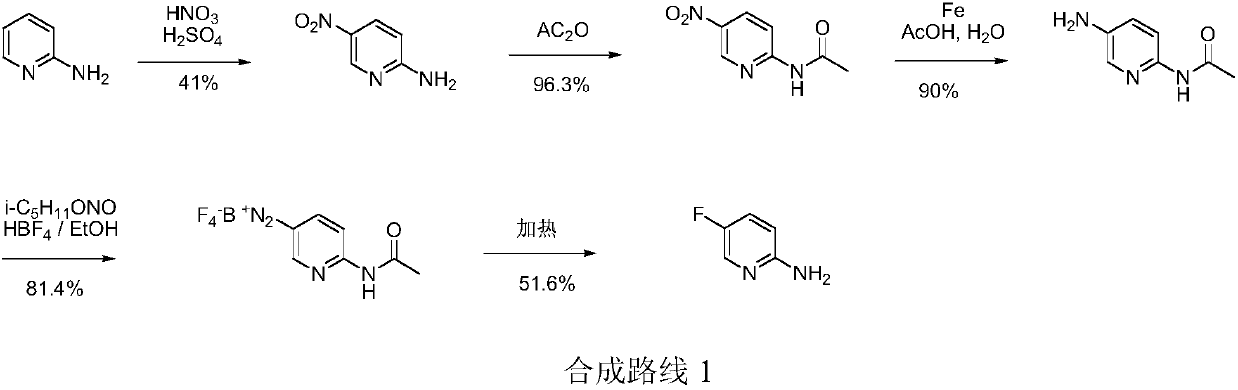
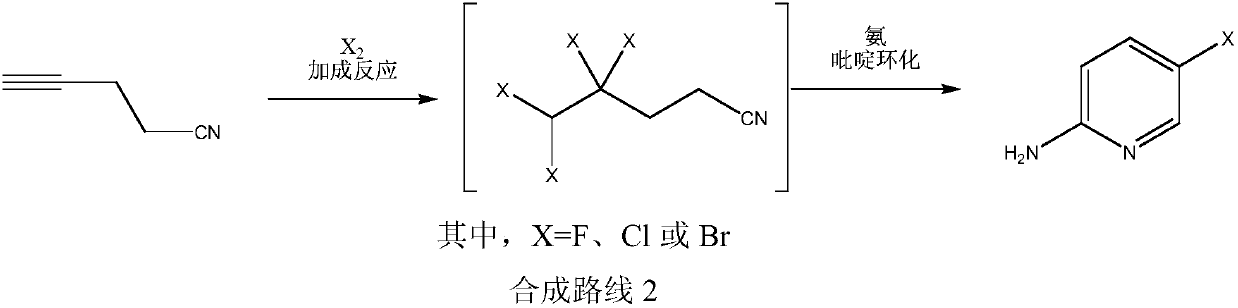
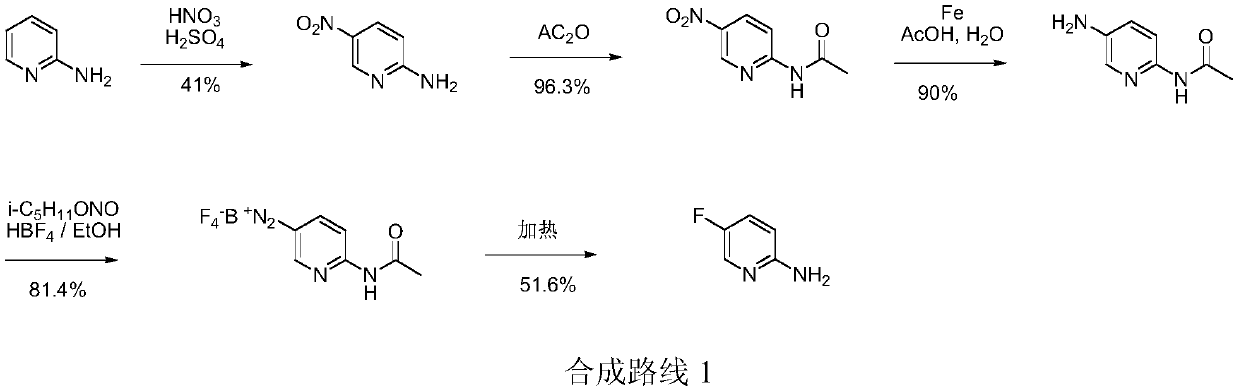
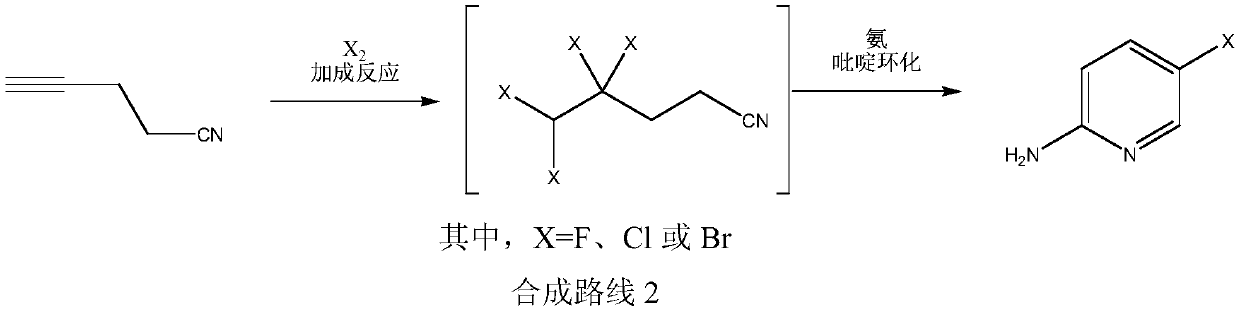
Simple 2-amino-5-halogenated pyridine preparation method
The invention provides a 2-amino-5-halogenated pyridine preparation method, which comprises: carrying out an addition reaction on 4-cyano-1-butyne and a halogen element X2 in a solvent under the catalysis of an acidic catalyst to obtain 4,4,5,5-tetrahalogenated n-valeronitrile, and carrying out cyclization on the 4,4,5,5-tetrahalogenated n-valeronitrile and ammonia through pyridine to obtain 2-amino-5-halogenated pyridine. According to the present invention, the preparation method has advantages of mild preparation condition, safety, environmental protection, low cost, high selectivity, less by-products and high product yield, and is suitable for industrial production.
Owner:XINFA PHARMA
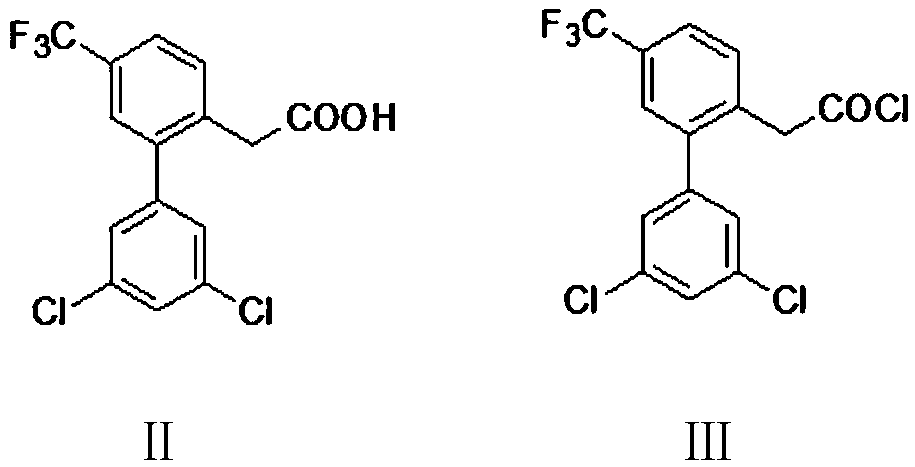
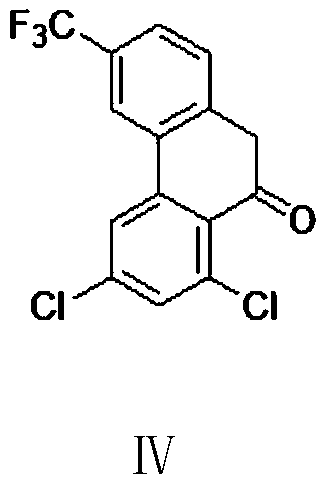
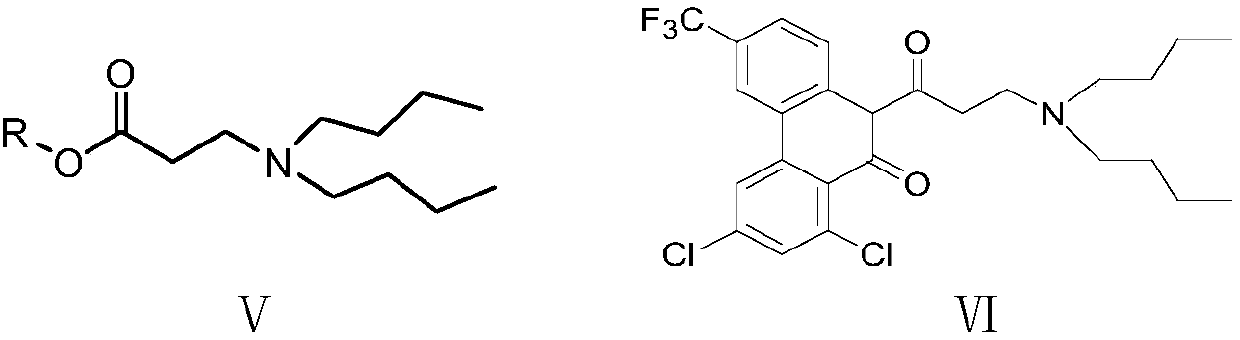
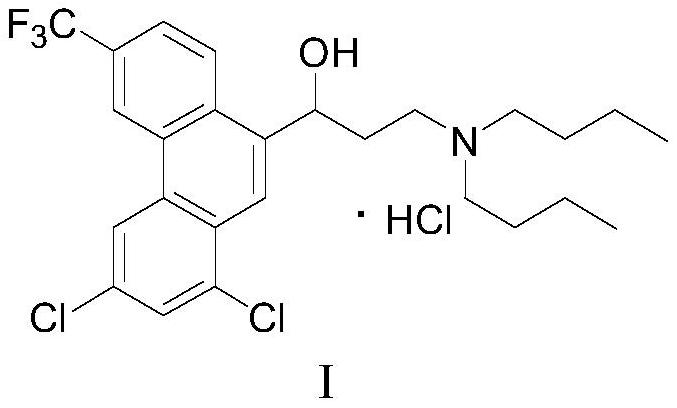
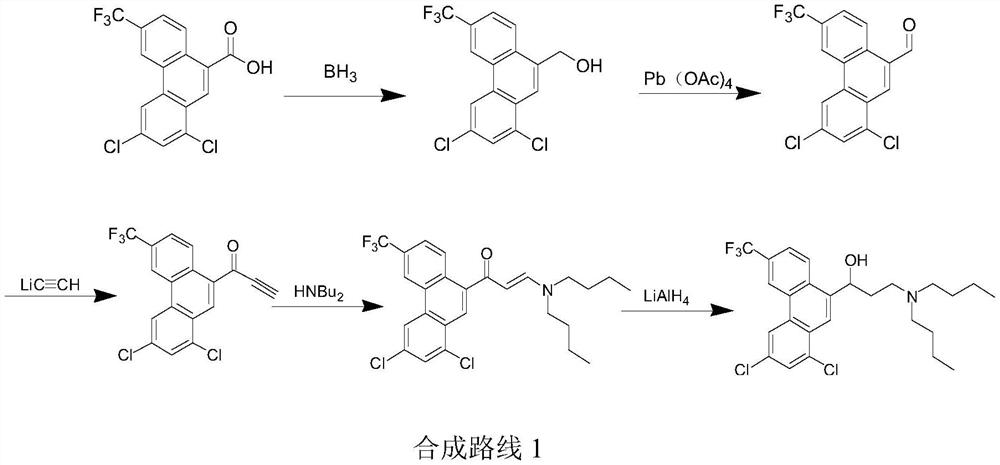
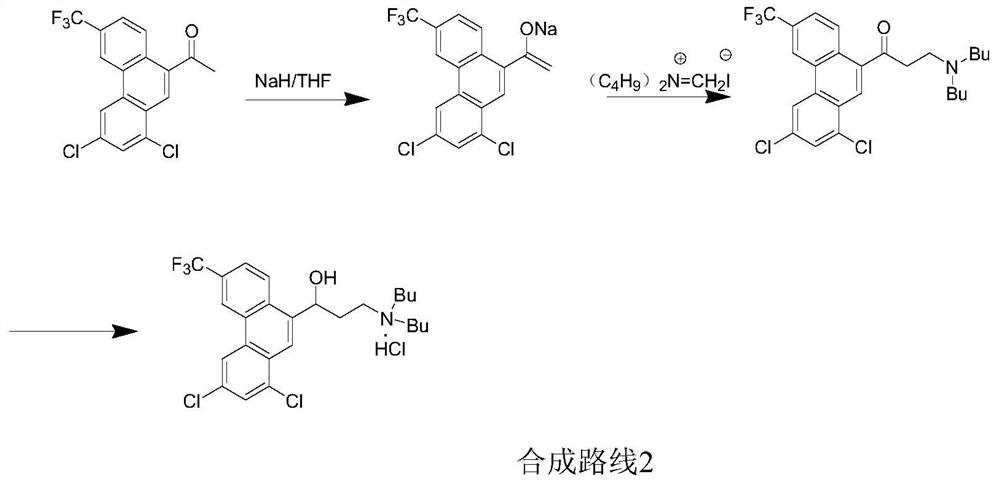
Preparation method of halofantrine hydrochloride
ActiveCN111484417AHigh yieldHigh purityOrganic compound preparationCarbonyl compound preparation by condensationPropanoic acidPhenylacetic acid
The invention provides a preparation method of halofantrine hydrochloride. The method comprises the following steps: generating corresponding acyl chloride from 4-trifluoromethyl-2-(3, 5-dichlorophenyl) phenylacetic acid and an acylating chlorination reagent, carrying out Friedel-Crafts reaction cyclization, performing condensation with N, N-di-n-butyl-beta-aminopropionate, and finally, carrying out reduction, aromatization and salification to obtain the halofantralin hydrochloride. The method has the advantages of cheap and accessible raw materials and low cost; reaction conditions are easy to realize, the technological process and operation are safe, simple and convenient, less wastewater is generated, and environmental friendliness is achieved; and the intermediate is good in stability,the reaction activity and selectivity are high, there are fewer side reactions, and the prepared halofantrine hydrochloride is few in impurity, high in purity and high in yield.
Owner:XINFA PHARMA
A kind of alectinib intermediate and the preparation method of alectinib
Owner:XINFA PHARMA
A kind of convenient preparation method of vemurafenib and its analogs
ActiveCN109970733BReduce productionConducive to green industrial productionOrganic chemistryWater productionAcyl group
The invention provides a simple preparation method of vemurafenib and analogues thereof. The method includes: subjecting para-substituted phenylacetaldehyde II and N-[2, 4-disubstituted-3-(cyanopropionyl)phenyl]n-propanesulfonamide III to azeotropic dewatering and condensation reaction under alkaili catalysis, condensing the obtained condensation product and the methylenation reagent V(N, N-dimethylformamide or tri-orthoformate), and then performing cyclization with ammonia to obtain vemurafenib or analogues thereof. The method for preparation of vemurafenib provided by the invention has the characteristics of low cost, mild process conditions, low operation requirements, short reaction time, high production efficiency, simple process operation, little waste water production, green and environmental protection, high yield and purity, and is beneficial to green industrial production of vemurafenib. At the same time, the method provided by the invention can prepare vemurafenib analogues,and is of important significance for the drug efficacy study of similar compounds.
Owner:XINFA PHARMA
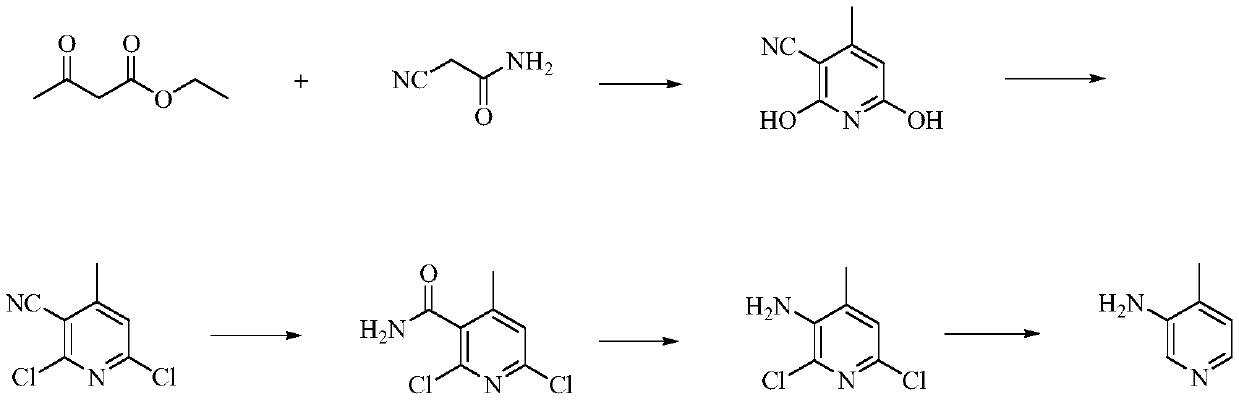
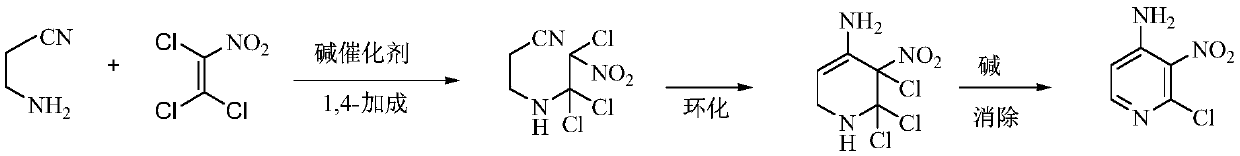
A kind of preparation method of 4-amino-2-chloro-3-nitropyridine
The invention relates to an environmental protection preparation method of 4-amino-2-chloro-3-nitropyridine. The method uses 3-amino propionitrile and trichloronitroethylene for 1,4-Addition and cyclization reaction to obtain 4-amino--2, 2, 3-trichloro-3-nitro-1, 2, 3, 6-tetrahydropyridine, and eliminates hydrogen chloride with an acid binding agent to prepare the 4-amino-2-chloro-3-nitropyridine.The method has the advantages of good reaction selectivity, good target product yield, high purity, short process flow, safe and simple operation, mild conditions, no waste acid and wastewater, environmental protection and low cost, and is carried out by a one-pot method.
Owner:XINFA PHARMA
A kind of preparation method of Halofantrine hydrochloride
ActiveCN111484417BHigh yieldHigh purityOrganic compound preparationCarbonyl compound preparation by condensationPropanoic acidPhenylacetic acid
Owner:XINFA PHARMA
A kind of convenient preparation method of 2-amino-5-halopyridine
ActiveCN110092746BReduce productionImprove economyOrganic chemistryPtru catalystEnvironmental engineering
The invention provides a 2-amino-5-halogenated pyridine preparation method, which comprises: carrying out an addition reaction on 4-cyano-1-butyne and a halogen element X2 in a solvent under the catalysis of an acidic catalyst to obtain 4,4,5,5-tetrahalogenated n-valeronitrile, and carrying out cyclization on the 4,4,5,5-tetrahalogenated n-valeronitrile and ammonia through pyridine to obtain 2-amino-5-halogenated pyridine. According to the present invention, the preparation method has advantages of mild preparation condition, safety, environmental protection, low cost, high selectivity, less by-products and high product yield, and is suitable for industrial production.
Owner:XINFA PHARMA
A kind of preparation method of pyridine derivative
ActiveCN110483383BRaw materials are cheap and easy to getShort routeOrganic chemistryKetoneEnvironmental engineering
The invention relates to a preparation method of pyridine derivatives. The method uses piperidine-4-one hydrochloride as a raw material to obtain a series of pyridine derivatives through halogenation reaction and elimination reaction. Preparation of 3,5-dihalopiperidin-4-one and 3,3,5-trihalogenated piperidine-4 through halogenation reaction with piperidine-4-one hydrochloride and a specific amount of halogenated reagent -ketone or 3,3,5,5-tetrahalogenated piperidine-4-ketone, and then react with different types of basic reagents to obtain pyridine derivatives whose 4-position is respectively hydroxyl, amino or dimethylamino Elimination reaction. The invention has the advantages of simple and convenient operation, mild conditions, short process flow, low waste water volume, environmental protection and low cost, and is beneficial to the green industrialized production of the pyridine derivatives.
Owner:XINFA PHARMA
A solvent-free coupling synthesis process of disperse blue 360
The invention discloses a solvent-free coupling synthesis process of disperse blue 360. 2-amino-5-nitrothiazole is added to an organic acid medium, and nitrosylsulfuric acid is added dropwise under temperature control to carry out diazotization reaction to obtain diazonium solution; add N,N-diethyl-m-methylaniline to the coupling reactor for ansolvation reaction, cool down to -10-10°C, add the above-mentioned diazo solution for coupling reaction until the coupling is completed, filter and wash to obtain Crude product; high-purity and finer blue crystals obtained through the crystallization process, that is, disperse blue 360. The solvent-free coupling synthesis process of disperse blue 360 provided by the present invention can obtain pure disperse blue 360 crystals with high yield, and the organic acid and crystal conversion solvent produced can all be recycled and used mechanically, saving energy and reducing waste, greatly reducing production costs , serve multiple purposes, and have very good practical significance for realizing large-scale clean production.
Owner:JIANGSU HANSYN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com