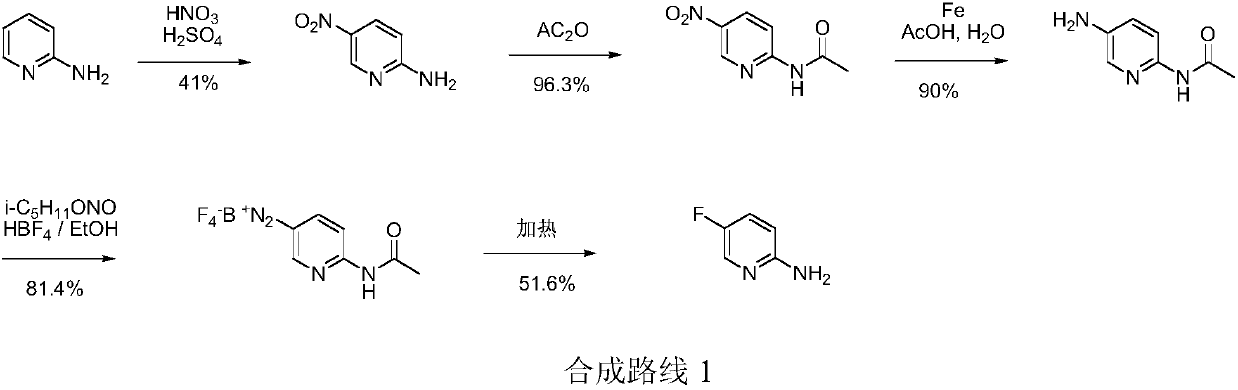
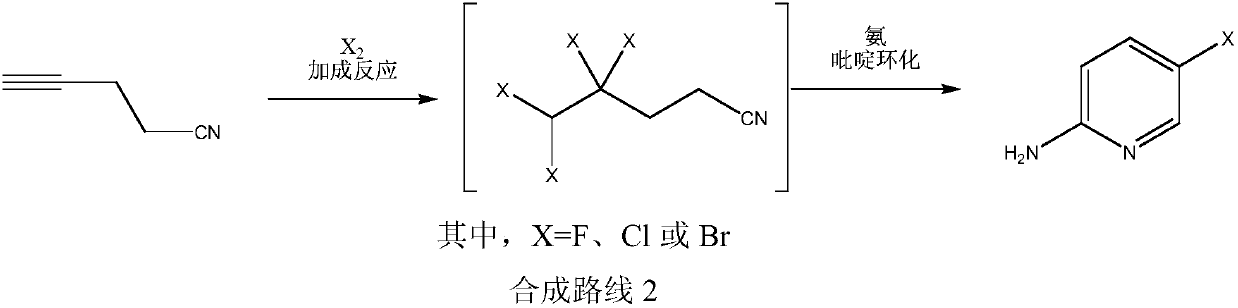
Simple 2-amino-5-halogenated pyridine preparation method
A technology of halopyridine and ammonia via pyridine, which is applied in the field of simple preparation of 2-amino-5-halopyridine, which can solve the problems of unfavorable industrial production application, easy flushing or even explosion, and high temperature control requirements, and achieve low cost , easy operation, high selectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: the preparation of 2-amino-5-chloropyridine
[0030] In the 500 milliliter four-neck flask that is connected with stirring, thermometer, reflux condenser and 20wt% sodium hydroxide aqueous solution tail gas absorption device, add 90 grams of 1,2-dichloroethane, 15.8 grams (0.2 moles) of 4-cyano -1-butyne, 0.2 g of ferric chloride (0.0012 mol), under stirring, intermittently feed chlorine gas at 40-45 ° C, and feed 30 g of chlorine gas in total for 2 hours, after ventilation is completed, stir and react at 40-45 ° C for 2 hours Nitrogen was then bubbled for 2 hours to replace residual chlorine and hydrogen chloride. Add 100 grams of 17wt% ammonia water, stir and react at 60-65°C for 5 hours, cool to 20°C, separate layers, extract the water layer three times with 1,2-dichloroethane, 20 grams each time, combine the organic phases, and use 20 1 g of saturated brine, then dried with 5 g of anhydrous sodium sulfate, and 1,2-dichloroethane was recovered by rotar...
Embodiment 2
[0033] Embodiment 2: the preparation of 2-amino-5-bromopyridine
[0034] In the 500 milliliter four-necked flask that is connected with stirring, thermometer, reflux condenser and 20wt% aqueous sodium hydroxide tail gas absorption device, add 90 grams of dichloromethane, 15.8 grams (0.2 moles) of 4-cyano-1-butyne , 0.3 grams of 40wt% hydrobromic acid aqueous solution (0.0015 moles), under stirring, dropwise add 65.0 grams of bromine and 50 grams of dichloromethane mixture at 30-35 ° C, dropwise for about 1 hour, and stir at 40-45 ° C for reaction 4 hours, then nitrogen bubbles for 2 hours to displace residual bromine and hydrogen bromide. Add 140 grams of 17wt% ammonia water, stir and react at 50-55°C for 4 hours, cool to 20°C, separate layers, extract the aqueous layer three times with dichloromethane, 20 grams each time, combine the organic phases, and wash with 20 grams of saturated brine , and then dried with 5 grams of anhydrous sodium sulfate, and the dichloromethane wa...
Embodiment 3
[0037] Embodiment 3: Preparation of 2-amino-5-fluoropyridine
[0038]Add 120 grams of 1,2-ethylene dichloride, 15.8 grams (0.2 mole) 4-cyano-1-butyne, 0.5 gram cuprous chloride (0.005 mole), under stirring, at 30-35 ℃ intermittently pass into the fluorine gas diluted with nitrogen (the mass concentration of fluorine gas is 2-5wt%), A total of 8 grams of fluorine gas was fed in equivalent to fluorine gas within 5 hours, then stirred and reacted at 35-40°C for 3 hours, and samples were taken at the same time to detect the completion of the addition reaction, and then nitrogen gas was bubbled for 2 hours to replace residual fluorine gas and hydrogen fluoride. Add 120 grams of 17wt% ammonia water, stir and react at 60-65°C for 5 hours, cool to 20°C, separate layers, extract the water layer three times with 1,2-dichloroethane, 20 grams each time, combine the organic phases, and use 20 Wash with 5 g of saturated brine, then dry with 5 g of anhydrous sodium sulfate, and recover 1,2-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com