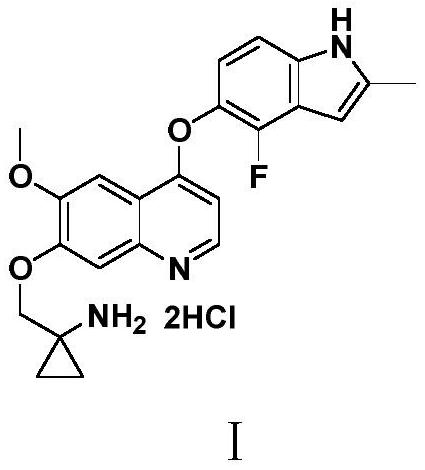
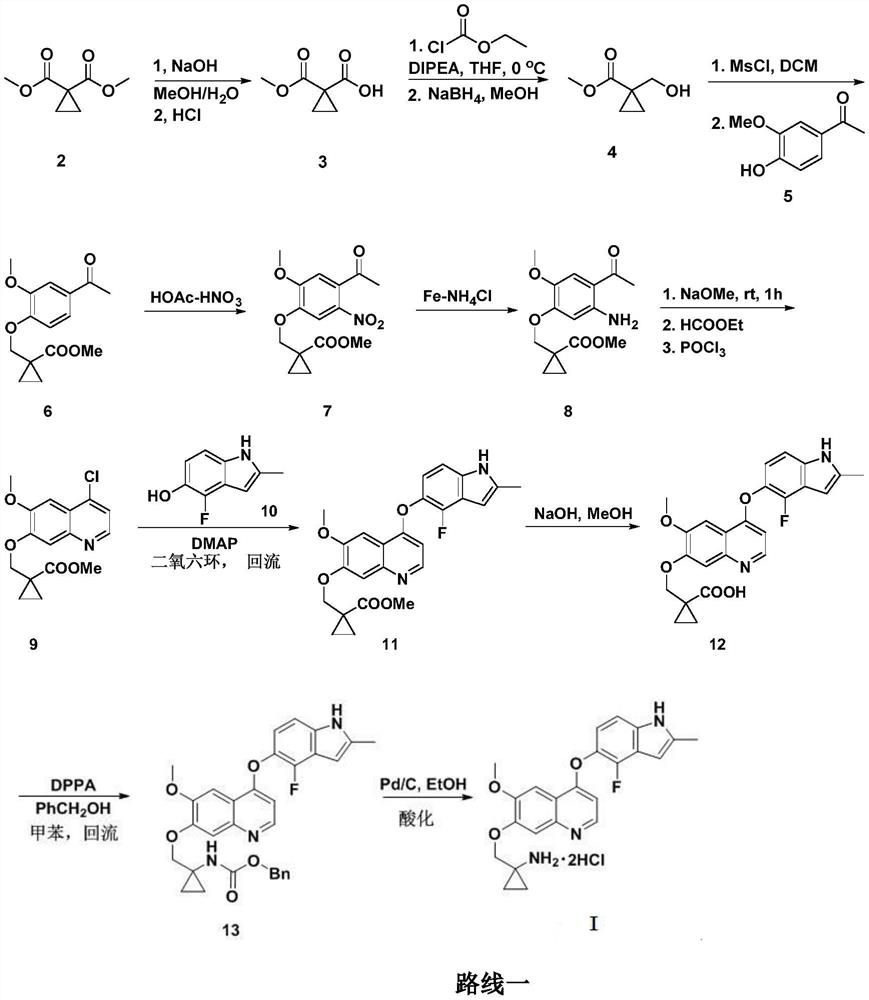
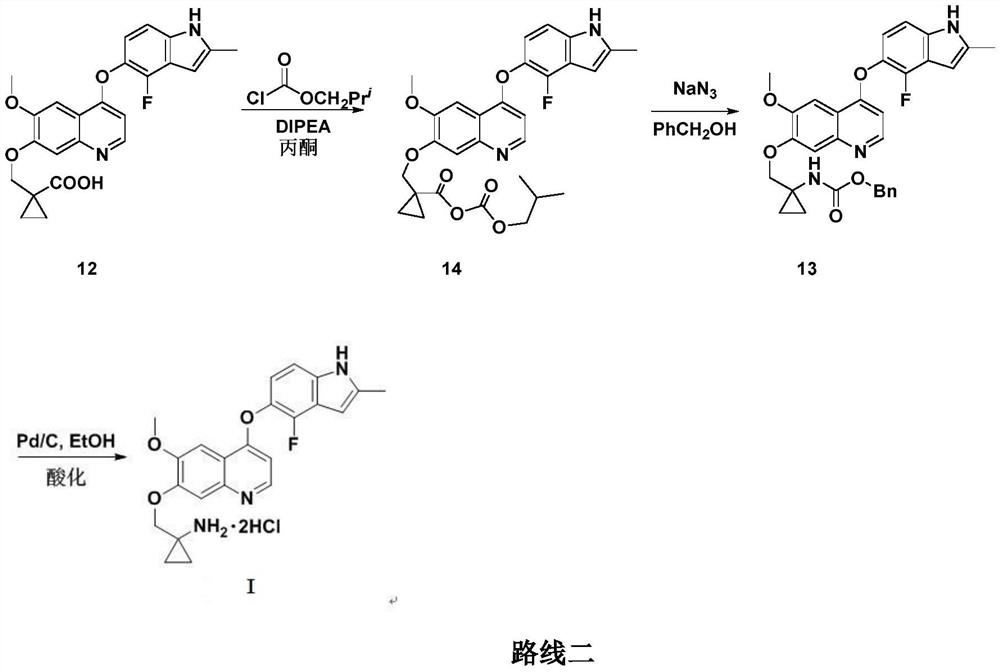
Anlotinib hydrochloride intermediate and preparation method of Anlotinib hydrochloride
A technology of anlotinib hydrochloride and intermediates, applied in the field of medicinal chemistry, can solve the problems of cumbersome operation, long steps and high price, and achieve the effect of short process flow, mild reaction conditions and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1: Preparation of 4-(2-fluoro-3-methyl-4-nitrophenyl)oxy-6-methoxy-7-benzyloxyquinoline (Ⅳ1)
[0066] To a 500 ml four-neck flask connected with stirring, a thermometer, and a reflux condenser, add 200 g of N,N-dimethylformamide, 28.0 g (0.1 mole) of 4-hydroxy-6-methoxy-7-benzyl Oxyquinoline (II1), 25.5 g (0.11 mol) 2-fluoro-3-bromo-6-nitrotoluene (III1), 19.5 g (0.14 mol) potassium carbonate, stirred at 95 to 100° C. for 3 hours. Cool to 20 to 25°C, filter to remove potassium salt, wash the filter cake with 30 grams of solvent, combine the filtrates, distill under reduced pressure to recover the solvent, then add 0.5 grams of activated carbon and 160 grams of 80% ethanol to the residue, react and stir at 80°C for decolorization For 1 hour, filter while hot, cool and recrystallize, filter and dry to obtain 39.6 grams of 4-(2-fluoro-3-methyl-4-nitrophenyl)oxy-6-methoxy-7-benzyloxy Quinoline, the yield is 91.2%, and the liquid phase purity is 99.7%.
Embodiment 2
[0067] Example 2: Preparation of 4-(2-fluoro-3-methyl-4-nitrophenyl)oxy-6-methoxy-7-benzyloxyquinoline (Ⅳ1)
[0068] In the 500 milliliter four-necked flask that is connected with stirring, thermometer, reflux condenser, add 200 gram tetrahydrofuran, 28.0 gram (0.1 mol) 4-hydroxyl-6-methoxy-7-benzyloxyquinoline (II1), 29.0 g (0.1 mole) of 2-fluoro-3-iodo-6-nitrotoluene (Ⅲ2), 20.7 g (0.15 mole) of potassium carbonate, and stirred at 65 to 70° C. for 6 hours. Cool to 20 to 25°C, filter to remove potassium salt, wash the filter cake with 30 grams of solvent, combine the filtrates, distill under reduced pressure to recover the solvent, then add 0.5 grams of activated carbon and 160 grams of 80% ethanol to the residue, react and stir at 80°C for decolorization For 1 hour, filter while hot, cool and recrystallize, filter, and dry to obtain 39.2 grams of 4-(2-fluoro-3-methyl-4-nitrophenyl)oxy-6-methoxy-7-benzyloxy Quinoline, the yield is 90.3%, and the liquid phase purity is 99.3%. ...
Embodiment 3
[0069] Example 3: 4-(2-fluoro-3-methyl-4-nitrophenyl)oxy-6-methoxy-7-(1-acetylaminocyclopropanyl-1-yl)methyloxy Preparation of Quinoline (Ⅳ2)
[0070] In a 500 ml four-neck flask connected with stirring, a thermometer, and a reflux condenser, add 200 g of N,N-dimethylformamide, 30.2 g (0.1 mole) of 4-hydroxyl-6-methoxyl-7-( 1-acetylaminocyclopropyl-1-yl) methyloxyquinoline (Ⅱ2), 25.7 grams (0.11 moles) 2-fluoro-3-bromo-6-nitrotoluene (Ⅲ1), 20.7 grams (0.15 moles ) Potassium carbonate, stirred and reacted at 95 to 100° C. for 3 hours. Cool to 20 to 25°C, filter to remove potassium salt, wash the filter cake with 30 grams of solvent, combine the filtrates, distill under reduced pressure to recover the solvent, then add 0.5 grams of activated carbon and 220 grams of 80% ethanol to the residue, react and stir at 80°C for decolorization For 1 hour, filter while hot, cool and recrystallize, filter and dry to obtain 40.9 grams of 4-(2-fluoro-3-methyl-4-nitrophenyl)oxy-6-methoxy-7-(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com