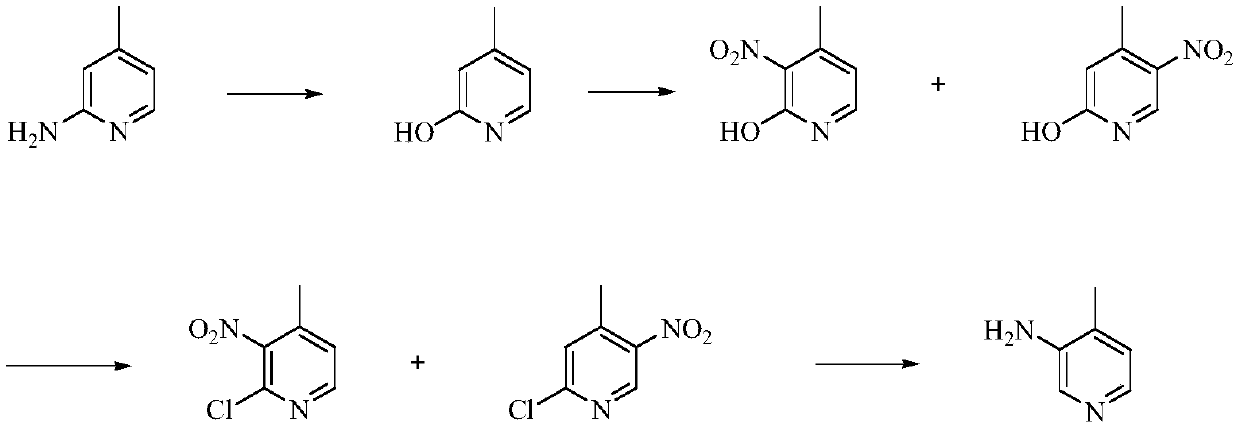
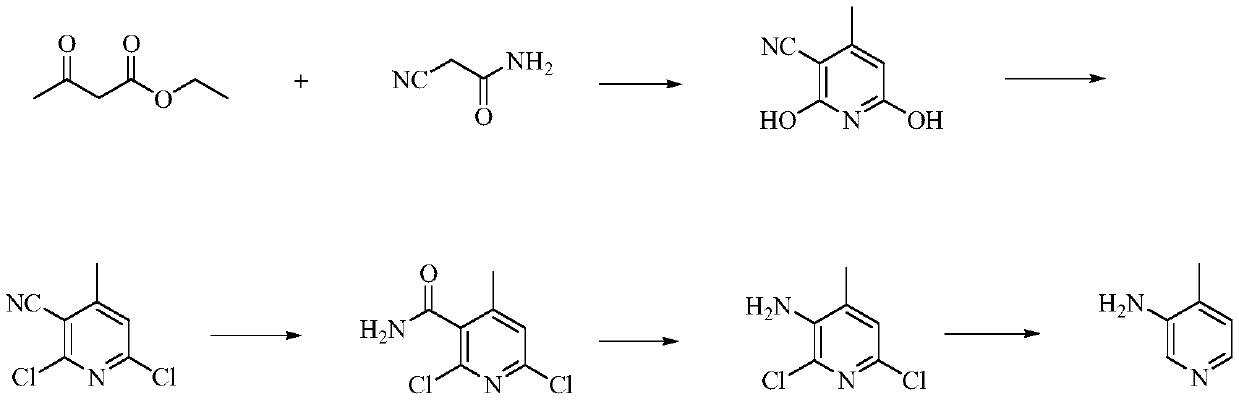
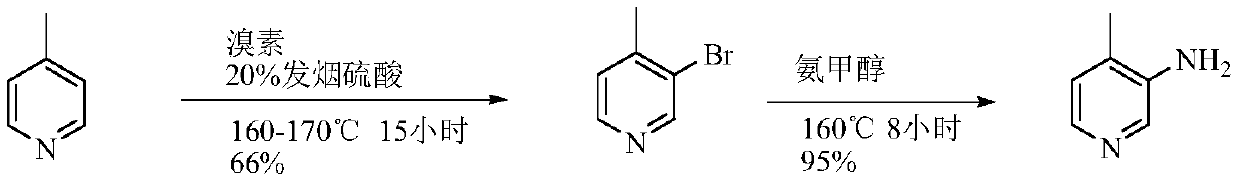
A kind of preparation method of 3-amino-4-picoline
A technology of picoline and fampridine, applied in the field of medicinal chemistry, can solve the problems of high price and high price of 4-picoline-3-boronic acid, and achieve the effects of easy post-processing, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: the preparation of 4-methyl-3-nitropyridine
[0056] Add 10.5 grams (0.1 moles) of 3-chlorocrotonaldehyde, 30.5 grams (0.5 moles) of nitromethane, and 0.4 grams of DBU to a 250-milliliter four-neck flask connected with a stirring, thermometer, and reflux condenser, and stir at 40-45°C React for 4 hours, after the reaction is completed by gas phase detection; add 17.5 grams (0.15 moles) of N,N-dimethylformamide dimethyl acetal, raise the temperature to 100-105 °C and stir for 4 hours, while steaming out and recovering excess nitromethane , After the gas phase detection reaction is completed; cool to 30°C, add 30.0 grams of 17% ammonia water, 50 grams of dichloromethane, stir and react at 30-35°C for 4 hours, cool to 20°C, separate layers, and extract the water layer with dichloromethane 3 times, 20 grams of dichloromethane each time, combined the organic phases, dried 3.0 grams of anhydrous sodium sulfate for 3 hours, filtered, and distilled the filtrate to...
Embodiment 2
[0057] Embodiment 2: the preparation of 4-methyl-3-nitropyridine
[0058] In a 500 ml four-neck flask connected with stirring, thermometer and reflux condenser, add 15.0 g (0.1 mole) of 3-bromocrotonaldehyde, 50 g of dichloromethane, 6.7 g (0.11 mole) of nitromethane, and 0.4 g of DBU , stirred and reacted at 40-45°C for 4 hours. After the reaction was completed by gas phase detection, 17.5 grams (0.15 moles) of N,N-dimethylformamide dimethyl acetal was added, and the temperature was raised to 100-105°C and stirred for 4 hours. Recover dichloromethane and excess nitromethane. After the reaction is completed by gas phase detection, cool to 30°C, add 35.0 grams of 15% ammonia-methanol solution, stir and react at 40-45°C for 4 hours, recover methanol by distillation under reduced pressure, and cool to 20 ℃, add 100 grams of water, 50 grams of dichloromethane, separate layers, extract the aqueous layer 3 times with dichloromethane, each time 20 grams of dichloromethane, combine th...
Embodiment 3
[0059] Embodiment 3: the preparation of 4-methyl-3-nitropyridine
[0060] In the 250 milliliters four-neck flasks that are connected with stirring, thermometer, reflux condenser, add 10.5 grams (0.1 moles) 2-chlorocrotonaldehyde, 50 grams toluene, 6.7 grams (0.11 moles) nitromethane, 0.5 grams piperidine, Stir and react at 40-45°C for 4 hours. After the reaction is completed by gas phase detection, add 17.5 g (0.15 moles) of N,N-dimethylformamide dimethyl acetal, raise the temperature to 100-105°C, stir and react for 4 hours, and simultaneously evaporate and recover Dichloromethane and excess nitromethane, after the gas phase detection reaction is completed, cool to 30 ° C, add 35.0 grams of 15% ammonia-methanol solution, stir and react at 40-45 ° C for 4 hours, recover methanol and toluene by distillation under reduced pressure, and cool to 20°C, add 100 grams of water and 50 grams of dichloromethane to the residue, separate layers, extract the aqueous layer with dichlorometh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com