Preparation method of halofantrine hydrochloride
A hydrochloride and halogen flooding technology, applied in the field of medicinal chemistry, can solve the problems of large amount of wastewater, high cost, and many impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
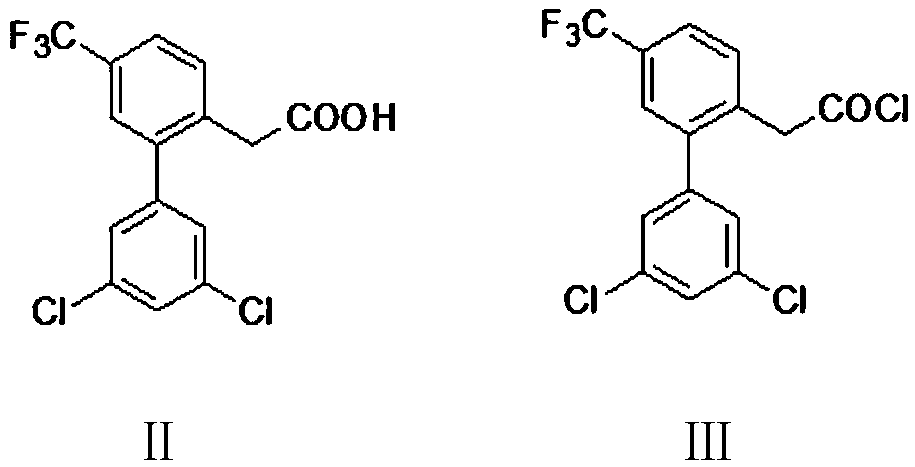
[0075] Example 1: Preparation of 4-trifluoromethyl-2-(3,5-dichlorophenyl)phenylacetyl chloride (Ⅲ)
[0076] Into a 500 ml four-necked flask equipped with a stirring, thermometer, reflux condenser and exhaust gas absorption device of 20% NaOH aqueous solution, 350 g of dichloromethane and 69.8 g (0.2 mole) of 4-trifluoromethyl-2-( 3,5-Dichlorophenyl)phenylacetic acid (II), 27.5g (0.23mol) of thionyl chloride was added dropwise at 20-30℃ for 1 hour, and then stirred and reacted at 35-40℃ for 4 hours, then distilled Recover solvent and excess thionyl chloride. 72.5 g of colorless transparent liquid 4-trifluoromethyl-2-(3,5-dichlorophenyl)phenylacetyl chloride (III) was obtained, with a gas phase purity of 99.6% and a yield of 98.2%.
Embodiment 2
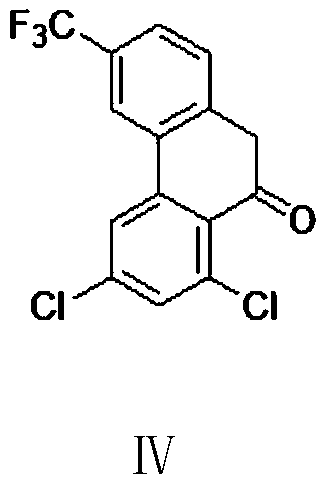
[0077] Example 2: Preparation of 3-trifluoromethyl-6,8-dichloro-9-one-10-hydrophenanthrene (IV)
[0078] Into a 500 ml four-necked flask connected with a stirring, thermometer, reflux condenser and exhaust gas absorption device of 20% NaOH aqueous solution, add 150 g of dichloromethane, 20.0 g (0.15 mole) of anhydrous aluminum trichloride, cool, and At 5-10°C, add dropwise a mixed solution of 36.7 g (0.1 mole) of 4-trifluoromethyl-2-(3,5-dichlorophenyl)phenylacetyl chloride (Ⅲ) obtained in Example 1 and 50 g of dichloromethane , The dripping was completed in 1 hour, after that, the reaction was stirred at 35 to 40°C for 3 hours. Cool to 0-5°C, slowly add the obtained liquid to 200 g of 3% hydrochloric acid at 0-5°C, separate the layers, and extract the aqueous layer twice with dichloromethane, each time with 50 g of dichloromethane. The organic phase was washed with 50 grams of saturated sodium bicarbonate aqueous solution and twice with pure water, 50 grams each time, the organ...
Embodiment 3
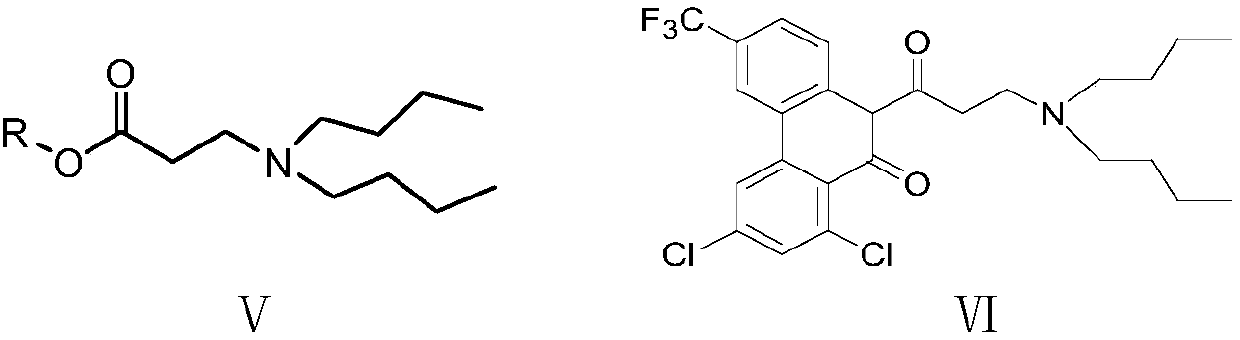
[0079] Example 3: 3-Trifluoromethyl-6,8-dichloro-9-one-10-[3-(di-n-butylamino)ethyl-1-keto]-10-hydrophenanthrene (VI ) Preparation
[0080] Into a 500 ml four-necked flask connected with a stirring, thermometer, and reflux condenser, add 200 g of methanol, 22.5 g (0.11 mol) of 27% sodium methoxide methanol solution, heat to 50 to 55°C, and add 33.1 dropwise at this temperature G (0.1 mole) of 3-trifluoromethyl-6,8-dichloro-9-one-10-hydrophenanthrene (IV) obtained in Example 2 and 21.5 g (0.1 mole) of N,N-di-n-butyl- Methyl β-alanine (V 1 ) And a mixed solution of 50 grams of methanol, the dripping is completed in 1 hour, and then kept at this temperature for 2 hours; the material is distilled under reduced pressure to evaporate the methanol, 50 grams of water and 200 grams of dichloromethane are added, and the pH value is neutralized with acetic acid For 5.0-6.0, separate the organic phase, extract the water layer twice with dichloromethane, 50 grams each time, combine the organ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com