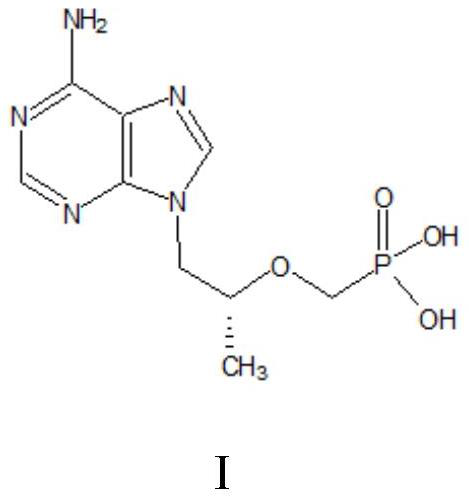
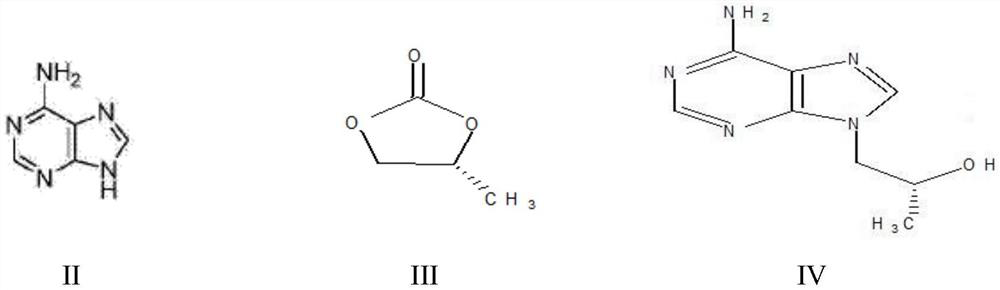
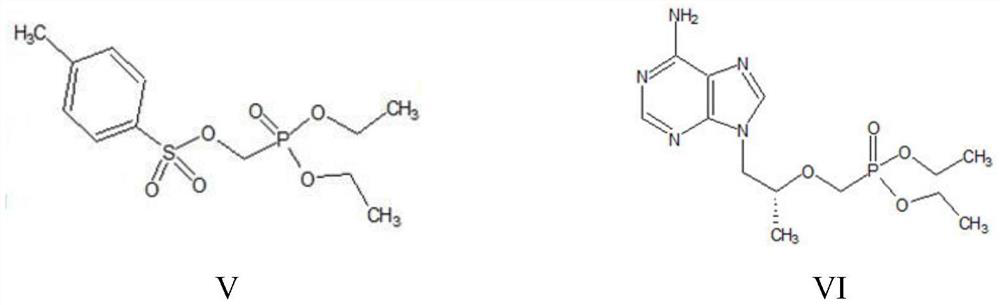
A kind of method utilizing microreactor to prepare tenofovir
A technology of microreactor and tenofovir, which is applied in the field of drug synthesis, can solve the problems of safety risks in operation, low yield, and large amount of waste liquid, and achieve low price, high yield and purity, and waste liquid generation. less effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] A kind of method utilizing microreactor to prepare tenofovir comprises steps:
[0055] (1) Dissolve 40g of adenine (II) and 39.32g of (R)-propylene carbonate (III) in 120g of DMF, add 2g of sodium hydroxide as a catalyst, mix well, protect with nitrogen, and heat up to 130°C for reaction 7h, liquid phase detection raw material residue is less than 0.5%; lower to room temperature, then add 160g toluene to crystallize, cool to 5°C, grow crystal for 1h, filter, wash, dry at 80°C for 7h to obtain (R)-9-(2-hydroxypropane Base) adenine (IV) 44g; product purity > 99%, molar yield 77%.
[0056] (2) (R)-9-(2-hydroxypropyl) adenine (IV), 30g magnesium tert-butoxide, 86.68g p-toluenesulfonyloxyphosphonic acid diethyl ester ( V) Dissolve in 120g DMF, react at 80°C for 5h, detect HPA (compound of formula IV) remaining less than 1%, add glacial acetic acid to quench, remove DMF by distillation under reduced pressure, add dichloromethane and water for extraction and layering, take T...
Embodiment 2
[0059] A kind of method utilizing microreactor to prepare tenofovir comprises steps:
[0060] (1) Dissolve 40g of adenine (II) and 39.32g of (R)-propylene carbonate (III) in 120g of NMP, add 2g of sodium hydroxide as a catalyst, mix well, protect with nitrogen, and heat up to 125°C for reaction 7h, liquid phase detection raw material residue is less than 0.5%; lower to room temperature, then add 160g ethanol to crystallize, cool to 5°C, grow crystal for 1h, filter, wash, dry at 80°C for 7h to obtain (R)-9-(2-hydroxypropane Base) adenine (IV) 40g; product purity > 99%, molar yield 70%.
[0061] (2) 40g of (R)-9-(2-hydroxypropyl)adenine (IV), 28g of magnesium tert-butoxide, 85g of diethyl p-toluenesulfonyloxyphosphonate (V) obtained in step (1) ) was dissolved in 120g of NMP, and after reacting at 80°C for 5h, it was detected that the remaining HPA was less than 1%. It was quenched by adding glacial acetic acid, and after the NMP was distilled off under reduced pressure, dichlo...
Embodiment 3
[0064] A kind of method utilizing microreactor to prepare tenofovir comprises steps:
[0065] (1) Dissolve 40g of adenine (II) and 39.32g of (R)-propylene carbonate (III) in 120g of DMF, add 2g of sodium hydroxide as a catalyst, mix well, protect with nitrogen, and heat up to 130°C for reaction 7h, liquid phase detection raw material residue is less than 0.5%; lower to room temperature, then add 160g toluene to crystallize, cool to 5°C, grow crystal for 1h, filter, wash, dry at 80°C for 7h to obtain (R)-9-(2-hydroxypropane Base) adenine (IV) 44g; product purity > 99%, molar yield 77%.
[0066] (2) (R)-9-(2-hydroxypropyl) adenine (IV), 30g magnesium tert-butoxide, 86.68g p-toluenesulfonyloxyphosphonic acid diethyl ester ( V) Dissolve in 120g DMF, react at 80°C for 5h, detect HPA remaining less than 1%, add glacial acetic acid to quench, remove DMF by distillation under reduced pressure, add dichloromethane and water to extract and separate layers, take the organic layer, and w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com