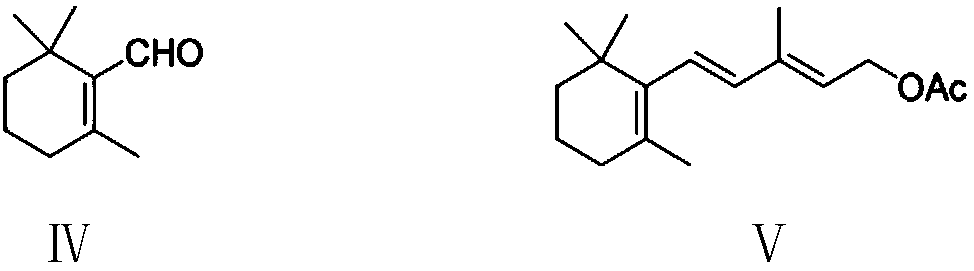
Preparation method of vitamin A acetate intermediate C15 and vitamin A acetate
The technology of acetate and vitamin is applied in the field of preparation of vitamin A acetate intermediate C15 and vitamin A acetate, and can solve the problems of unenvironmental protection, complicated post-treatment, large amount of waste water and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Embodiment 1: Formula III 1 Compound preparation
[0107] Formula III 1 The structural formula of the compound is as follows:
[0108]
[0109] Under nitrogen protection, add 250 grams of acetonitrile, 41.5 grams (0.2 moles) of 1-bromo-2-methyl-4-acetoxy-2-butyl alkene (II 1 ), 52.5 grams (0.2 moles) of triphenylphosphine, stirred and reacted at 60-65°C for 4 hours, cooled to 10-15°C, filtered, and obtained 89.2 grams of formula III after drying the filter cake 1 Compound, the filtrate was calibrated with a liquid-phase external standard method to have a triphenylphosphine content of 2.63 grams, which can be directly applied to the next batch of reactions. The calculated yield based on the actual conversion of triphenylphosphine was 99.9%, and the liquid-phase purity was 99.7%.
Embodiment 2
[0110] Embodiment 2: Formula III 2 Compound preparation
[0111] Formula III 2 The structural formula of the compound is as follows:
[0112]
[0113] Under nitrogen protection, add 250 grams of acetonitrile, 34.1 grams (0.21 moles) of 1-chloro-2-methyl-4-acetoxy-2-butyl alkene (II 2 ), 52.5 grams (0.2 moles) of triphenylphosphine, stirred and reacted at 70-75°C for 4 hours, cooled to 10-15°C, filtered, and obtained 77.8 grams of formula III after drying the filter cake 2 Compound, the filtrate was calibrated with a liquid-phase external standard method to have a triphenylphosphine content of 4.46 grams, which can be directly applied to the next batch of reactions. The calculated yield based on the actual conversion of triphenylphosphine was 99.9%, and the liquid-phase purity was 99.5%.
Embodiment 3
[0114] Embodiment 3: Formula III 3 Preparation of compound (diethyl 2-methyl-4-acetoxy-2-butenylphosphonate)
[0115] Formula III 3 The structural formula of the compound is as follows:
[0116]
[0117] Under nitrogen protection, add 41.5 grams (0.2 moles) of 1-bromo-2-methyl-4-acetoxy-2-butene (II 1 ), 41.6 grams (0.25 moles) of triethyl phosphite, stirred and reacted at 105-110°C for 4 hours (using ethanol to recover the generated by-product bromoethane), cooled to 70-75°C, replaced with a vacuum distillation device, and recovered After excessive triethyl phosphite, further underpressure distillation (110-120 ℃ / 1-2mmHg) obtains 49.6 grams of 2-methyl-4-acetoxy-2-butenyl phosphonic acid diethyl ester (Ⅲ 3 ), the yield is 93.9%, and the gas phase purity is 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com