Simple preparation method of vemurafenib and analogues thereof
A technology of compounds and meanings, applied in the field of medicinal chemistry, can solve the problems of complicated operation, long production cycle, low purity, etc., and achieve the effects of simple process operation, less waste water production, and high yield and purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
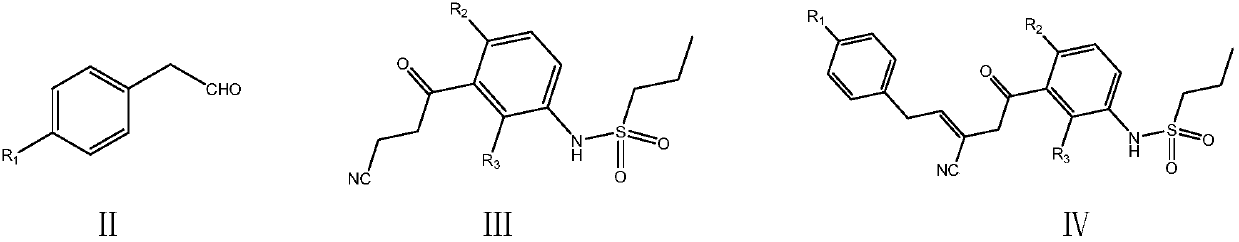
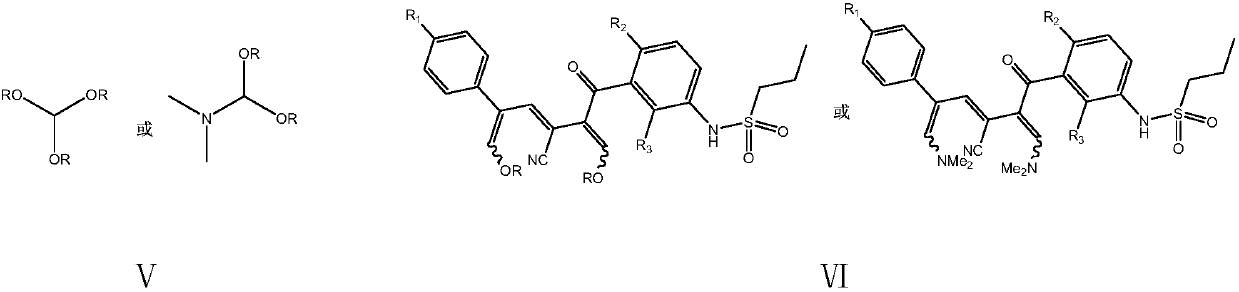
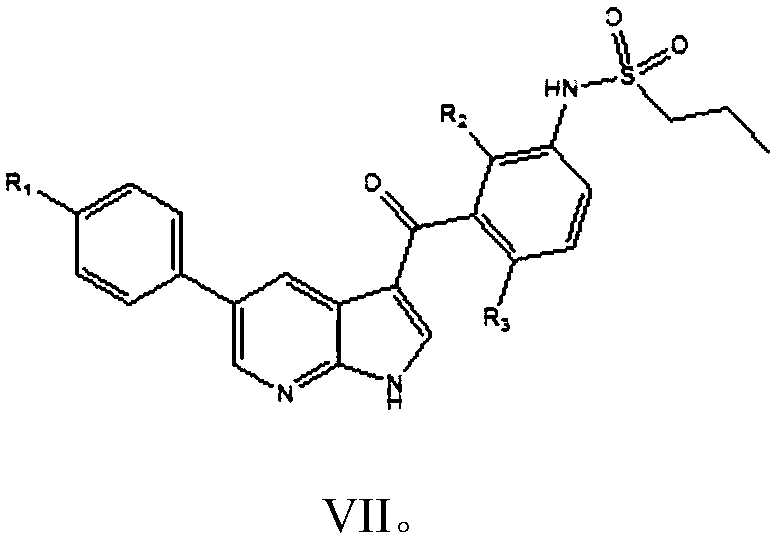
[0058] Embodiment 1: the preparation of vemurafenib (I)
[0059] In a 500 ml four-necked flask connected with stirring, a thermometer, and a reflux condenser with a water separator, add 250 grams of toluene, 31.5 grams (0.10 moles) of N-[2,4-difluoro-3-(cyano N-propionyl) phenyl] n-propanesulfonamide, 17.0 grams (0.11 moles) p-chlorophenylacetaldehyde, 0.5 grams of piperidine, heating, stirring, 83-85 ° C azeotropic reflux with water condensation reaction for 5 hours; cooling to 30 ℃, add 38.0 g (0.32 moles) of N,N-dimethylformamide dimethyl acetal, and stir at 85-90°C for 6 hours; cool to 30°C, add 70.0 g of 10wt% ammonia-methanol solution, and stir at 50-55°C React for 4 hours. Recover methanol and toluene by distillation under reduced pressure, add 200 grams of isopropanol to the residue, heat to 80 ° C, cool, recrystallize, filter, and dry to obtain 43.9 grams of vemurafenib, with a yield of 89.6% and a liquid phase purity of 99.91 %.
[0060] The NMR data of the produc...
Embodiment 2
[0063] Embodiment 2: the preparation of vemurafenib (I)
[0064] Add 300 g of 1,2-dichloroethane, 31.5 g (0.10 moles) of N-[2,4-bis Fluoro-3-(cyano-n-propionyl)phenyl]n-propanesulfonamide, 17.0 g (0.11 mole) p-chlorophenylacetaldehyde, 0.5 g DBU, heating, stirring, 71-73 ° C azeotropic reflux with water condensation reaction 5 hours; cooled to 30°C, added 38.0 g (0.32 moles) of N,N-dimethylformamide dimethyl acetal, stirred and reacted at 90-95°C for 5 hours, while recovering 1,2-dichloroethane; cooled to 30° C., 70.0 g of 10 wt % ammonia methanol solution was added to the residue, and the mixture was stirred and reacted at 50-55° C. for 4 hours. Recover methanol and 1,2-dichloroethane by distillation under reduced pressure, add 200 grams of isopropanol to the residue, heat to 80 ° C, cool, recrystallize, filter, and dry to obtain 44.7 grams of vemurafenib, yield 91.2%, liquid phase purity 99.89%.
Embodiment 3
[0065] Embodiment 3: the preparation of vemurafenib (I)
[0066] In a 500 ml four-necked flask connected with a stirring, thermometer, and a reflux condenser with a water separator, add 250 g of cyclohexane, 31.5 g (0.10 moles) of N-[2,4-difluoro-3-( Cyanopropionyl) phenyl] n-propanesulfonamide, 17.0 grams (0.11 moles) p-chlorophenylacetaldehyde, 0.5 grams of DBU, heating, stirring, 68-70 ° C azeotropic reflux with water condensation reaction for 8 hours; cooling to 30°C, add 5.0g (0.33 moles) of trimethyl orthoformate, 2.5g of anhydrous zinc chloride, stir and react at 60-65°C for 8 hours; cool to 30°C, add 70.0g of 10wt% ammonia methanol solution, 50-55 The reaction was stirred at °C for 5 hours. Recover cyclohexane and methanol by distillation under reduced pressure, add 300 grams of 80 wt% aqueous isopropanol to the residue, heat to 80 ° C, cool, recrystallize, filter, and dry to obtain 43.5 grams of vemurafenib, yield 88.8% , Liquid phase purity 99.79%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com