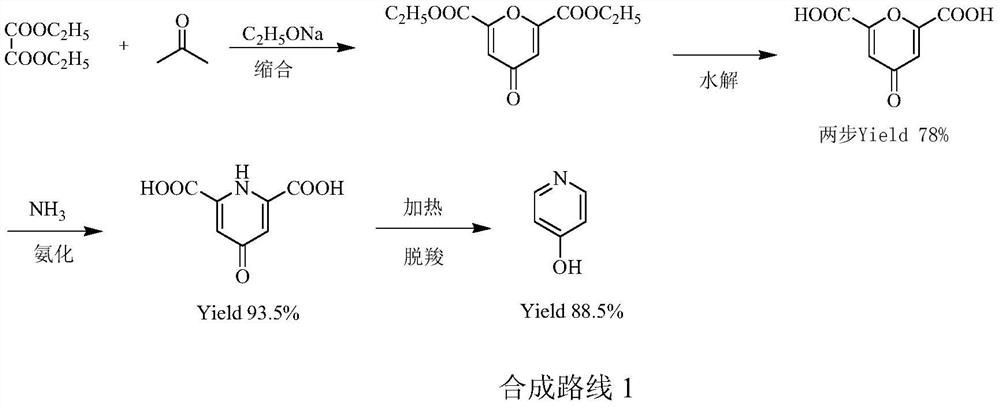
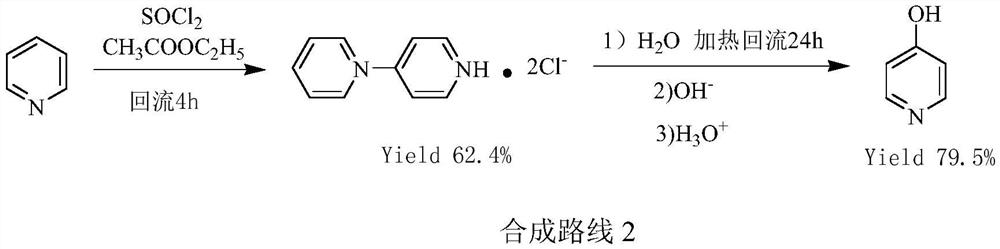
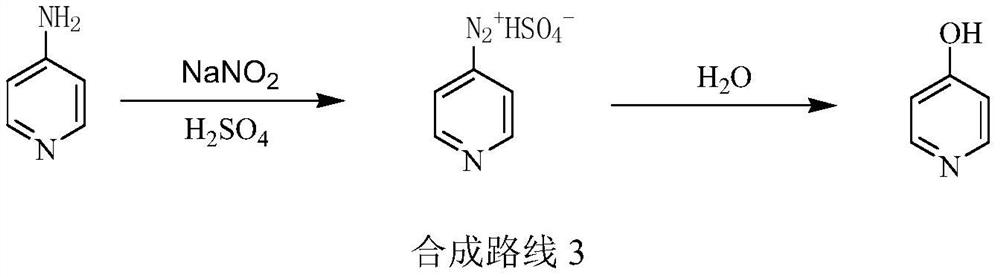
A kind of preparation method of pyridine derivative
A derivative, pyridine technology, applied in the field of preparation of pyridine derivatives, can solve problems such as complex process, high cost of raw materials, unfavorable environmental protection and industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1: the preparation of 3,5-dichloropiperidin-4-one
[0064] In a 500 ml four-neck flask connected with stirring, a thermometer, and a reflux condenser, 200 g of chloroform, 27.1 g (0.2 moles) of piperidin-4-one hydrochloride, and chlorine gas were passed between 40-45 ° C, and a total of Chlorine 32.0 grams, 40-45 ° C stirring reaction for 5 hours, cooled to 20-25 ° C, nitrogen to blow off residual chlorine and by-product hydrogen chloride gas, blow off for 2 hours, add 50 grams of water, adjust pH with 20% sodium carbonate aqueous solution The value is 7-8, layered, the organic phase is washed once with 20 grams of saturated aqueous sodium chloride solution, layered, and the solvent is recovered by distillation to obtain 39.9 grams of light yellow liquid 3,5-dichloropiperidin-4-one, directly for relevant elimination reactions.
Embodiment 2
[0065] Embodiment 2: Preparation of 3,5-dibromopiperidin-4-one
[0066] In the 500 milliliter four-necked flask that is connected with stirring, thermometer, reflux condenser, 200 grams of chloroform, 13.6 grams (0.1 moles) piperidin-4-one hydrochloride, 41.0 grams (0.2 moles) 40% hydrobromic acid, Add 24.0 (0.21 moles) of 30% hydrogen peroxide dropwise at 30-35°C for about 2 hours, stir and react at 30-35°C for 3 hours, add 50 grams of water, adjust the pH value to 7-8 with 20% aqueous sodium carbonate solution, Separate the layers, wash the organic phase once with 20 g of saturated aqueous sodium chloride solution, separate the layers, and recover the solvent by distillation to obtain 29.2 g of yellow liquid 3,5-dibromopiperidin-4-one, which is directly used in related elimination reactions.
Embodiment 3
[0067] Embodiment 3: Preparation of 3,3,5-trichloropiperidin-4-one
[0068] In the 500 milliliter four-necked flask that is connected with stirring, thermometer, reflux condenser, 200 gram dichloromethanes, 27.1 gram (0.2 moles) piperidin-4-one hydrochloride, 43.0 grams (0.41 moles) 35% hydrochloric acid, Add 74.0 (0.65 mole) 30% hydrogen peroxide dropwise at 30-35°C for about 3 hours, after that, stir and react at 40-45°C for 3 hours, cool to 20-25°C, blow off residual chlorine and hydrogen chloride gas for 1 hour with nitrogen , add 50 grams of water, adjust the pH value to 7-8 with 20% aqueous sodium carbonate solution, separate layers, wash the organic phase once with 20 grams of saturated aqueous sodium chloride solution, separate layers, and recycle the solvent by distillation to obtain 47.4 grams of yellow liquid 3 ,3,5-Trichloropiperidin-4-one, directly used in related elimination reactions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com