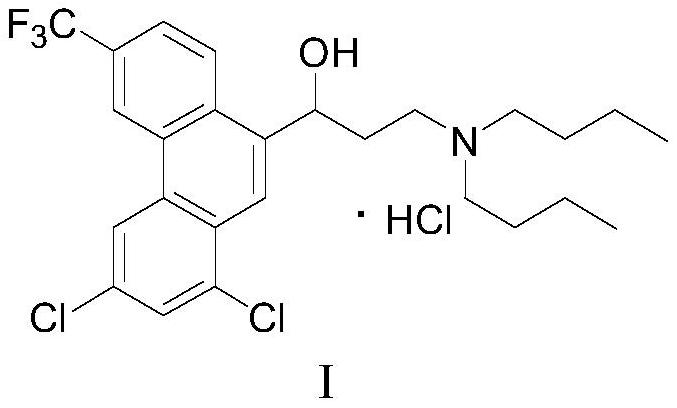
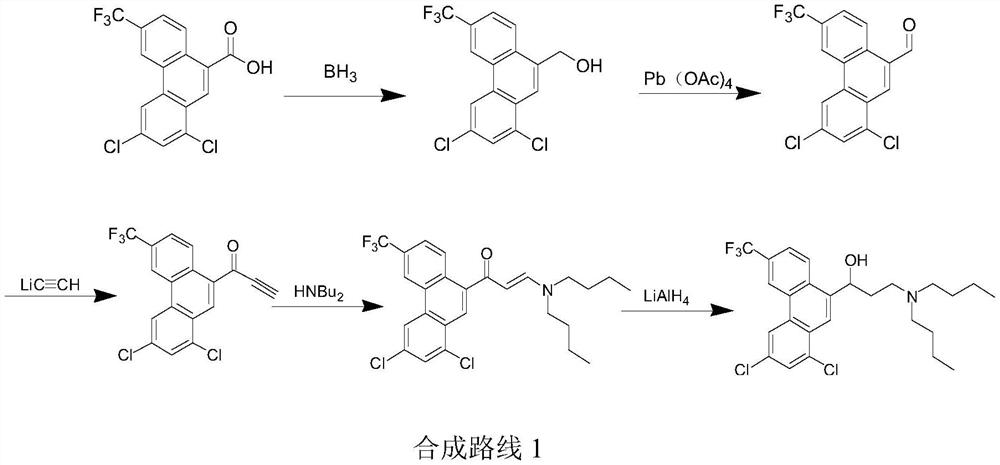
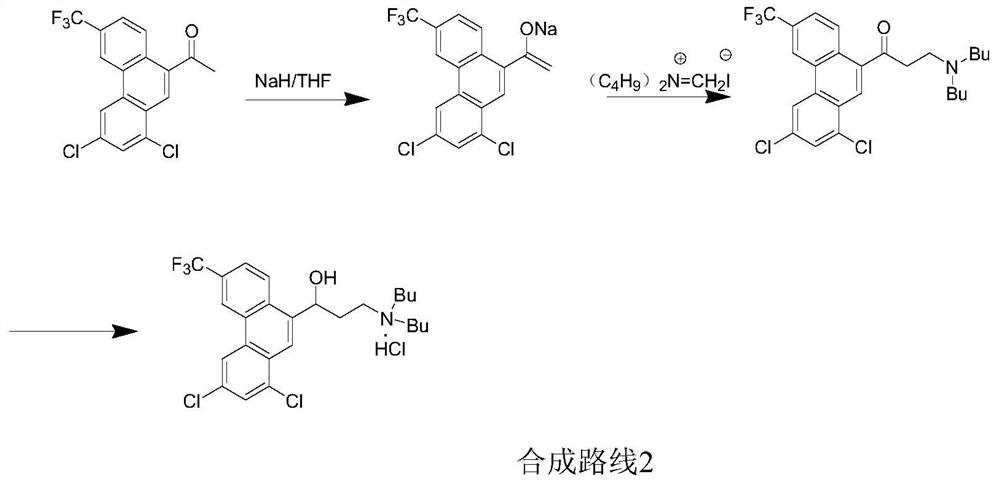
A kind of preparation method of Halofantrine hydrochloride
A technology of hydrochloride and halide, applied in the field of medicinal chemistry, can solve the problems of low free radical reaction yield, difficult industrial production, low reaction safety, etc., and achieves easy realization of reaction conditions, high reactivity and selectivity, The effect of reaction selectivity and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075]Example 1: Preparation of 4-trifluoromethyl-2-(3,5-dichlorophenyl) phenylacetyl chloride (III.).
[0076] To a 500 ml four-mouth flask equipped with a stirring, thermometer, reflux condensing tube and a tail gas absorption device with a 20% NaOH aqueous solution, 350 g of dichloromethane, 69.8 g (0.2 moles) 4-trifluoromethyl-2- (3,5-dichlorophenyl) phenylacetic acid (II.), 20-30 °C dropwise add 27.5 g (0.23 moles) sulfoxide chloride, drop addition time 1 hour, thereafter 35-40 °C stirring reaction for 4 hours, distillation recovery of solvent and excess sulfoxide chloride. To give 72.5 g of colorless transparent liquid 4-trifluoromethyl-2-(3,5-dichlorophenyl)phenylacetyl chloride (III.), gas-phase purity of 99.6%, yield of 98.2%.
Embodiment 2
[0077] Example 2: Preparation of 3-trifluoromethyl-6,8-dichloro-9-one-10-hydrophenanthrene (IV.).
[0078] To a 500 ml four-mouth flask connected to a stirring, the thermometer, reflux condensing tube and a 20% NaOH aqueous solution of the tail gas absorption apparatus, 150 g of dichloromethane, 20.0 g (0.15 moles) of anhydrous aluminum trichloride, cooled, 5-10 ° C dropwise added 36.7 g (0.1 mole) embodiment 1 to obtain 4-trifluoromethyl-2- (3,5-dichlorophenyl) phenylacetyl chloride (III.) and 50 g dichloromethane mixed solution, 1 hour dropwise added, thereafter, Stir the reaction at 35 to 40 °C for 3 h. Cooled to 0-5 ° C, the resulting liquid is slowly added to 0-5 ° C of 200 g of 3% hydrochloric acid, layered, the aqueous layer with dichloromethane extracted twice, each time 50 g of dichloromethane, the combined organic phase in turn with 50 g of saturated sodium bicarbonate aqueous solution washed once, pure water washed 2 times, 50 g each time, organic phase distillation to ...
Embodiment 3
[0079] Example 3: Preparation of 3-trifluoromethyl-6,8-dichloro-9-one-10-[3-(di-n-butylamine)ethyl-1-keto]-10-hydrophenanthrene (VI.).
[0080]To a stirring, the thermometer, reflux condensing tube of 500 ml four-mouth flask, add 200 g of methanol, 22.5 g (0.11 moles) 27% sodium methanol methanol solution, heated to 50 to 55 ° C, at this temperature added dropwise 33.1 g (0.1 moles) embodiment 2 to give 3-trifluoromethyl -6,8-dichloro-9-keto-10-hydrophenanthrene (IV.) and 21.5 g (0.1 moles) N, N- di-n-butyl - β - methyl propionate (V). 1 and a mixed solution of 50 grams of methanol, added dropwise in 1 hour, and then kept warm at this temperature for 2 hours; the material is distilled and distilled dry methanol under reduced pressure, adding 50 grams of water and 200 grams of dichloromethane, Neutralized pH with acetic acid of 5.0-6.0, the organic phase was separated, the aqueous layer was extracted with dichloromethane twice, 50 g each time, the organic phase was combined, the d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com