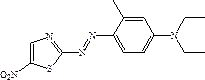
Solvent-free coupling synthesis process of disperse blue 360
A synthesis process, disperse blue technology, applied in the direction of azo dyes, monoazo dyes, organic dyes, etc., can solve the problems that acid waste liquid cannot be recycled, acetone and organic acid cannot be recovered separately, and the yield is only low, etc., to achieve Reduce the production of waste solvents and impurity components, facilitate recycling and improve yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
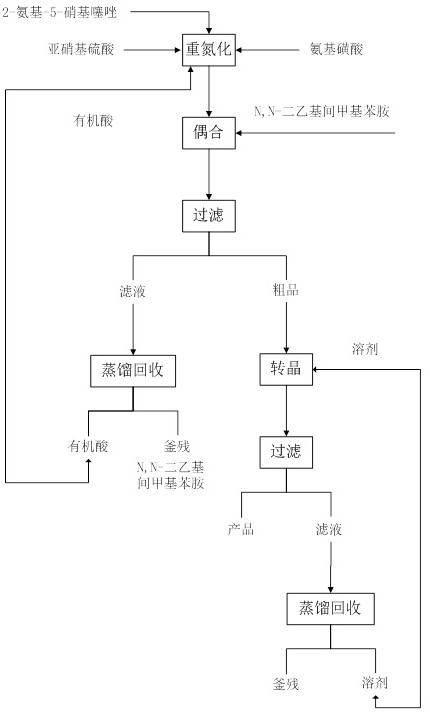
[0035] Put 200g (1.378mol, 1.0eq) of 2-amino-5-nitrothiazole and 2L organic acid into a 3L reaction flask with mechanical stirring (the volume ratio of acetic acid: propionic acid is 1:1.0), and then cool down to - 5°C, control the temperature at -5°C and slowly add 460g (1.447mol, 1.05eq) of 40% nitrosyl sulfuric acid dropwise, the system gradually changes from milky white to orange-yellow turbid liquid, the reaction is stirred for 3 hours, and the TLC plate (EA :PE=1:2), ultraviolet color development shows that the conversion of 2-amino-5-nitrothiazole is complete, add 14g (0.14mol, 0.1eq) sulfamic acid to quench the diazotization reagent, and prepare the diazonium solution for use ; Prepare another 3L reaction bottle with mechanical stirring, put 270g (1.654mol, 1.2eq) of N,N-diethyl-m-methylaniline, cool down to -5°C, and control the temperature at -5~5°C The above diazo solution was added dropwise to N,N-diethyl-m-methylaniline for coupling reaction, and the temperature w...
Embodiment 2
[0039] Put 200g (1.378mol, 1.0eq) of 2-amino-5-nitrothiazole and 2L organic acid into a 3L reaction flask with mechanical stirring (the volume ratio of acetic acid: propionic acid is 1:1.5), and then cool down to - 5°C, control the temperature at -5°C and slowly add 460g (1.447mol, 1.05eq) of 40% nitrosyl sulfuric acid dropwise, the system gradually changes from milky white to orange-yellow turbid liquid, the reaction is stirred for 3 hours, and the TLC plate (EA :PE=1:2), ultraviolet color development shows that the conversion of 2-amino-5-nitrothiazole is complete, add 14g (0.14mol, 0.1eq) sulfamic acid to quench the diazotization reagent, and prepare the diazonium solution for use ; Prepare another 3L reaction bottle with mechanical stirring, put 270g (1.654mol, 1.2eq) of N,N-diethyl-m-methylaniline, cool down to -5°C, and control the temperature at -5~5°C The above diazo solution was added dropwise to N,N-diethyl-m-methylaniline for coupling reaction, and the temperature w...
experiment example 3
[0043] Put 200g (1.378mol, 1.0eq) of 2-amino-5-nitrothiazole and 2L organic acid into a 3L reaction flask with mechanical stirring (the volume ratio of acetic acid: propionic acid is 1:2.0), and then cool down to - 5°C, control the temperature at -5°C and slowly add 460g (1.447mol, 1.05eq) of 40% nitrosyl sulfuric acid dropwise, the system gradually changes from milky white to orange-yellow turbid liquid, the reaction is stirred for 3 hours, and the TLC plate (EA :PE=1:2), ultraviolet color development shows that the conversion of 2-amino-5-nitrothiazole is complete, add 14g (0.14mol, 0.1eq) sulfamic acid to quench the diazotization reagent, and prepare the diazonium solution for use ; Prepare another 3L reaction bottle with mechanical stirring, put 270g (1.654mol, 1.2eq) of N,N-diethyl-m-methylaniline, cool down to -5°C, and control the temperature at -5~5°C The above diazo solution was added dropwise to N,N-diethyl-m-methylaniline for coupling reaction, and the temperature w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com