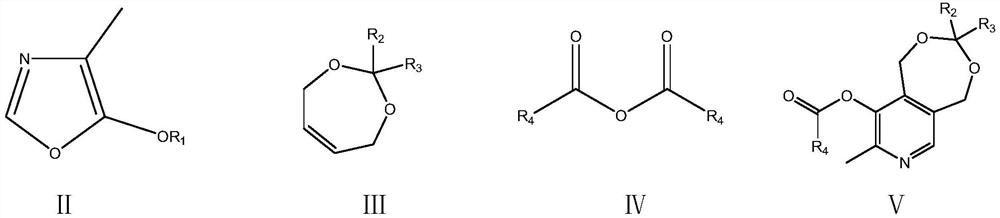
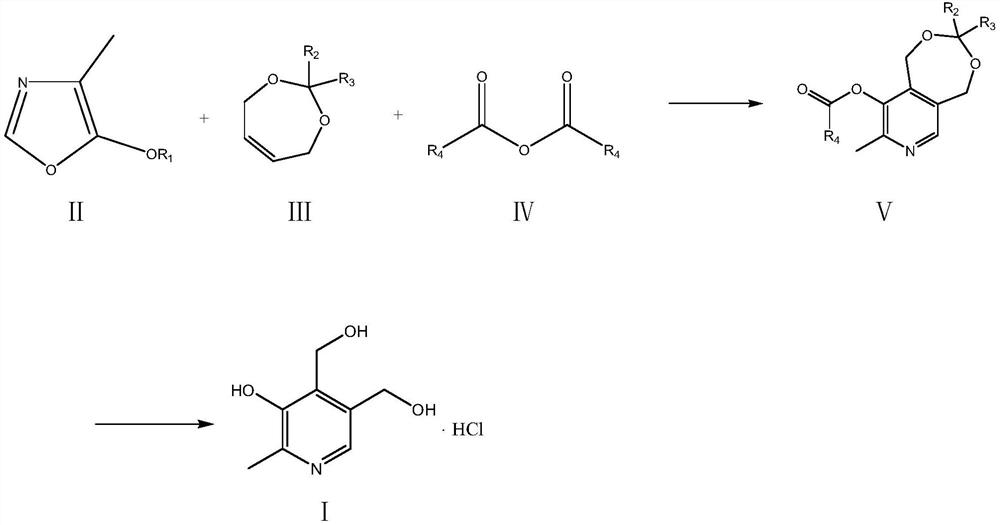
A kind of preparation method of vitamin B6
A vitamin and methyl technology, which is applied in the field of preparation of high-content vitamin B6, can solve the problems that the content of vitamin B is difficult to meet the medicinal standard, it is difficult to completely remove by-products, and the color of the product is heavy, so as to avoid multiple recrystallization and decolorization The effect of operation, high product yield and thorough reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
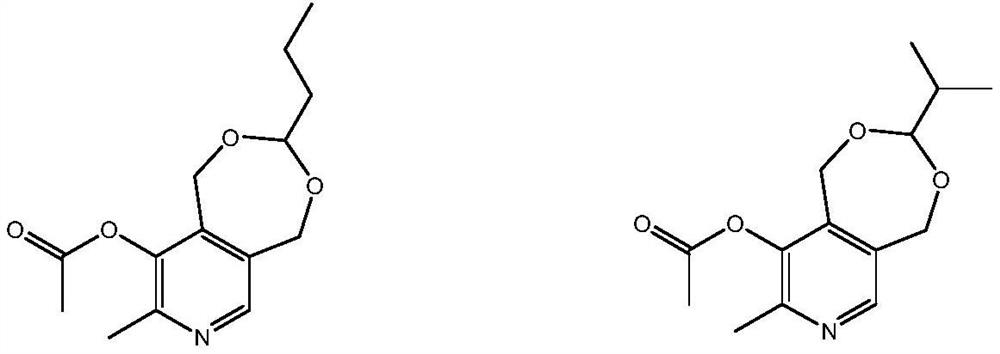
[0034] Example 1: 1,5-dihydro-3-n-propyl-8-methyl-9-methylcarbonyloxypyrido[3,4-e]-1,3-dioxane (V1) preparation of
[0035] Add 63.5 g (0.5 mol) of 4-methyl-5-ethoxyoxazole, 285.0 g (2.0 mol) of 2-n-propyl-4,7-dihydro-1,3- Dioxheptin, 60 g (0.6 moles) of acetic anhydride, replaced by nitrogen three times, heated to an internal temperature of 135-145° C., stirred for 8 hours, and the reaction pressure was 0.2-0.3 MPa. Lower the temperature to 50-60°C, and recover by-product ethanol, acetic acid, a small amount of acetic anhydride and excess 2-n-propyl-4,7-dihydro-1,3-dioxepin (separated by rectification) After ethanol and acetic acid, it can be used directly), and the product was collected by distillation at 160-180°C / 2-3mmHg under high vacuum to obtain 129.2 grams of 1,5-dihydro-3-n-propyl-8-methyl-9-methanol Carbonyloxypyrido[3,4-e]-1,3-dioxane (V1), yield 97.5%, HPLC purity 99.9%.
Embodiment 2
[0036] Example 2: 1,5-dihydro-3-n-propyl-8-methyl-9-methylcarbonyloxypyrido[3,4-e]-1,3-dioxane (V1) preparation of
[0037] Add 56.5 g (0.5 mol) of 4-methyl-5-methoxyoxazole, 285.0 g (2.0 mol) of 2-n-propyl-4,7-dihydro-1,3- Dioxheptin, 71.5 g (0.7 moles) of acetic anhydride, replaced by nitrogen three times, heated to an internal temperature of 130-135° C., stirred for 12 hours, and the reaction pressure was 0.3-0.4 MPa. Lower the temperature to 50-60°C, and recover by-product methanol, acetic acid, a small amount of acetic anhydride and excess 2-n-propyl-4,7-dihydro-1,3-dioxepin by distillation under reduced pressure (separated by rectification Methanol and acetic acid can be used directly), and the product was collected by distillation under high vacuum (160-180°C / 2-3mmHg) to obtain 129.0 g of 1,5-dihydro-3-n-propyl-8-methyl-9 -Methylcarbonyloxypyrido[3,4-e]-1,3-dioxane (V1), yield 97.4%, HPLC purity 99.9%.
Embodiment 3
[0038] Example 3: 1,5-dihydro-3-n-propyl-8-methyl-9-methylcarbonyloxypyrido[3,4-e]-1,3-dioxane (V1) preparation of
[0039] Add 63.5 g (0.5 mol) of 4-methyl-5-ethoxyoxazole, 142.5 g (1.0 mol) of 2-n-propyl-4,7-dihydro-1,3- Dioxheptin, 60.0 g (0.6 moles) of acetic anhydride, replaced with nitrogen three times, heated to an internal temperature of 135-145° C., stirred for 9 hours, and the reaction pressure was 0.2-0.3 MPa. Cool down to 50-60°C, and recover by-product ethanol, acetic acid, a small amount of acetic anhydride, unreacted 4-methyl-5-ethoxyoxazole, 2-n-propyl-4,7-dihydro by vacuum distillation -1,3-Dioxheptin (can be used directly after separation of ethanol and acetic acid by rectification), the product was collected by distillation at 160-180℃ / 2-3mmHg under high vacuum to obtain 86.8 grams of 1,5-dihydro- 3-n-propyl-8-methyl-9-methylcarbonyloxypyrido[3,4-e]-1,3-dioxane (V1), yield 95.5% (based on the actual consumption of 4 -methyl-5-ethoxyoxazole), the HPLC puri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com