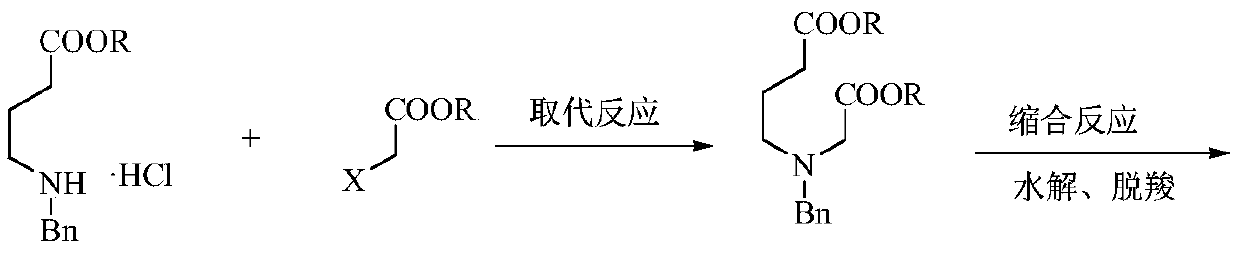
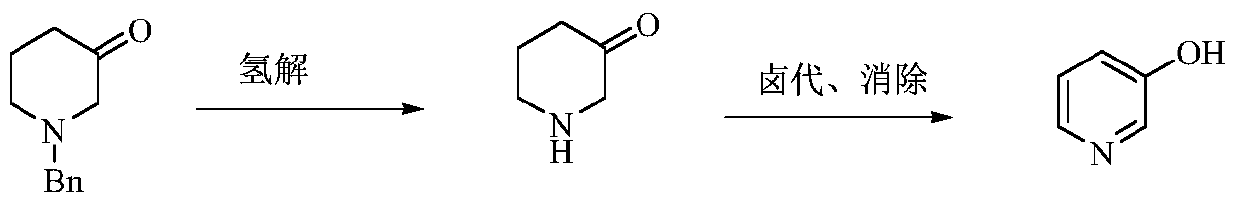
A kind of preparation method of low-cost 3-hydroxypyridine
A hydroxypyridine and benzyl technology, applied in the field of preparation of 3-hydroxypyridine, can solve the problems of unsuitability for industrial production, difficult operation, large amount of waste water, etc., and achieves easy implementation and control, reduced raw material cost, and good reaction selectivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Preparation of N-benzyl-3-aza-1,7-pimelic acid dimethyl ester
[0052] In the 500 milliliter four-necked flask that is connected with stirring, thermometer, reflux condenser, add 250 grams of methanol, 24.4 grams (0.1 moles) of methyl 4-benzylamino butyrate hydrochloride, 11.5 grams (0.105 moles) of 2- Methyl chloroacetate, 30.3 grams (0.22 moles) of potassium carbonate, stirred and reacted between 60-65°C for 6 hours, then cooled to 30°C, filtered, the filter cake was washed once with 40 grams of methanol, the filtrates were combined, and methanol was recovered by distillation , Distilled under reduced pressure (105-120° C. / 3 mmHg) to obtain 25.8 g of N-benzyl-3-aza-1,7-pimelic acid dimethyl ester with a yield of 92.6% and a gas phase purity of 99.5%.
Embodiment 2
[0053] Example 2: Preparation of N-benzyl-3-aza-1,7-pimelic acid dimethyl ester
[0054] In the 1000 milliliter four-necked flask that is connected with stirring, thermometer, reflux condenser, add 400 gram tetrahydrofuran, 49.0 gram (0.2 mol) methyl 4-benzylamino butyrate hydrochloride, 32.1 gram (0.21 mol) 2- Methyl bromoacetate, 58.0 g (0.42 moles) of potassium carbonate, stirred and reacted at 60-65°C for 6 hours, then cooled to 30°C, filtered, the filter cake was washed once with 40 g of tetrahydrofuran, the filtrates were combined, and tetrahydrofuran was recovered by distillation , Distilled under reduced pressure (105-120° C. / 3 mmHg) to obtain 52.6 g of N-benzyl-3-aza-1,7-pimelic acid dimethyl ester with a yield of 94.3% and a gas phase purity of 99.7%.
Embodiment 3
[0055] Example 3: Preparation of N-benzyl-3-aza-1,7-pimelic acid diethyl ester
[0056] In the 500 milliliter four-neck flask that is connected with stirring, thermometer, reflux condenser, add 200 grams of ethanol, 25.8 grams (0.1 moles) of ethyl 4-benzylaminobutyrate hydrochloride, 18.5 grams (0.11 moles) of 2- Ethyl bromoacetate, 26.5 grams (0.25 moles) of sodium carbonate, stirred and reacted between 70-75°C for 4 hours, then cooled to 30°C, filtered, the filter cake was washed with 40 grams of ethanol, the filtrates were combined, and the ethanol was recovered by distillation. Pressure distillation (105-125° C. / 3 mmHg) yielded 28.2 g of N-benzyl-3-aza-1,7-pimelic acid diethyl ester with a yield of 91.7% and a gas phase purity of 99.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com