Patents
Literature
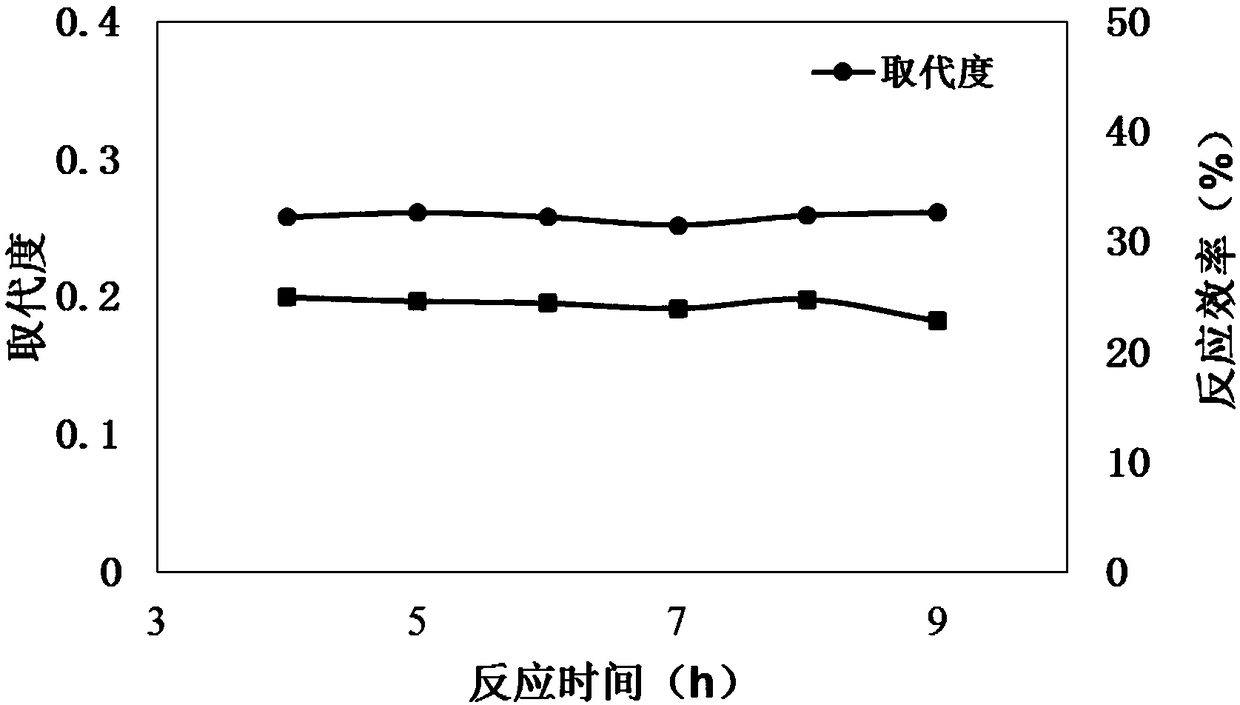
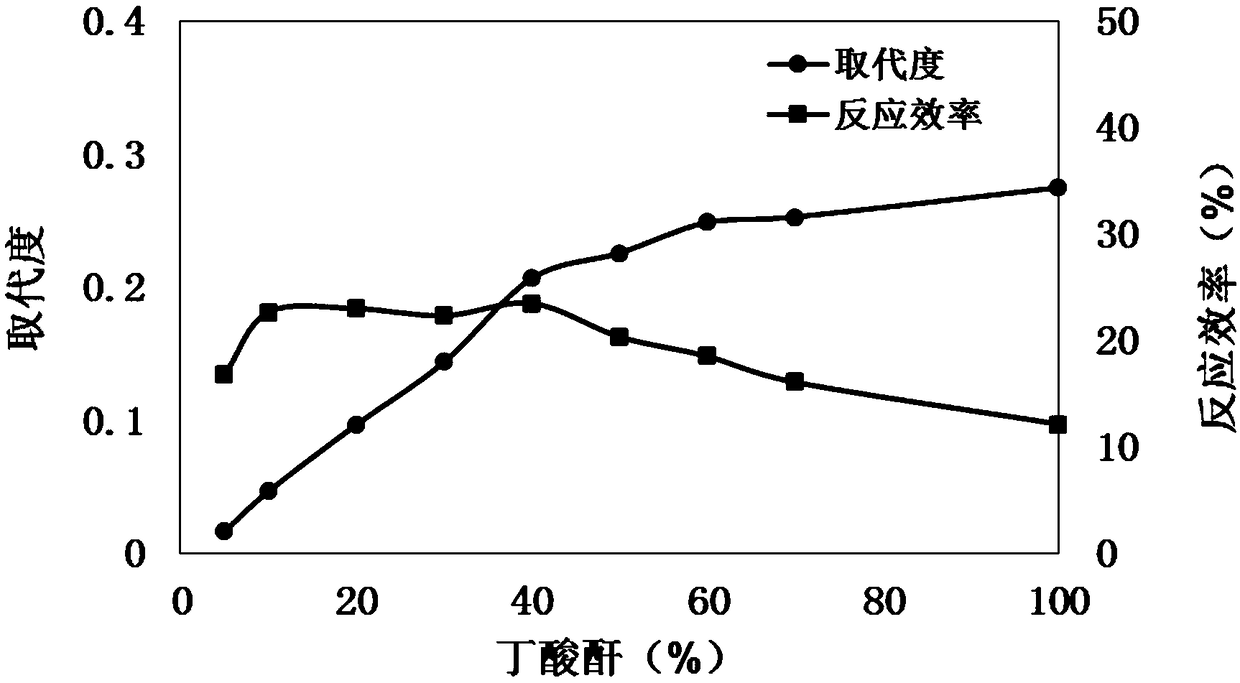
119 results about "Butyric acid ester" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
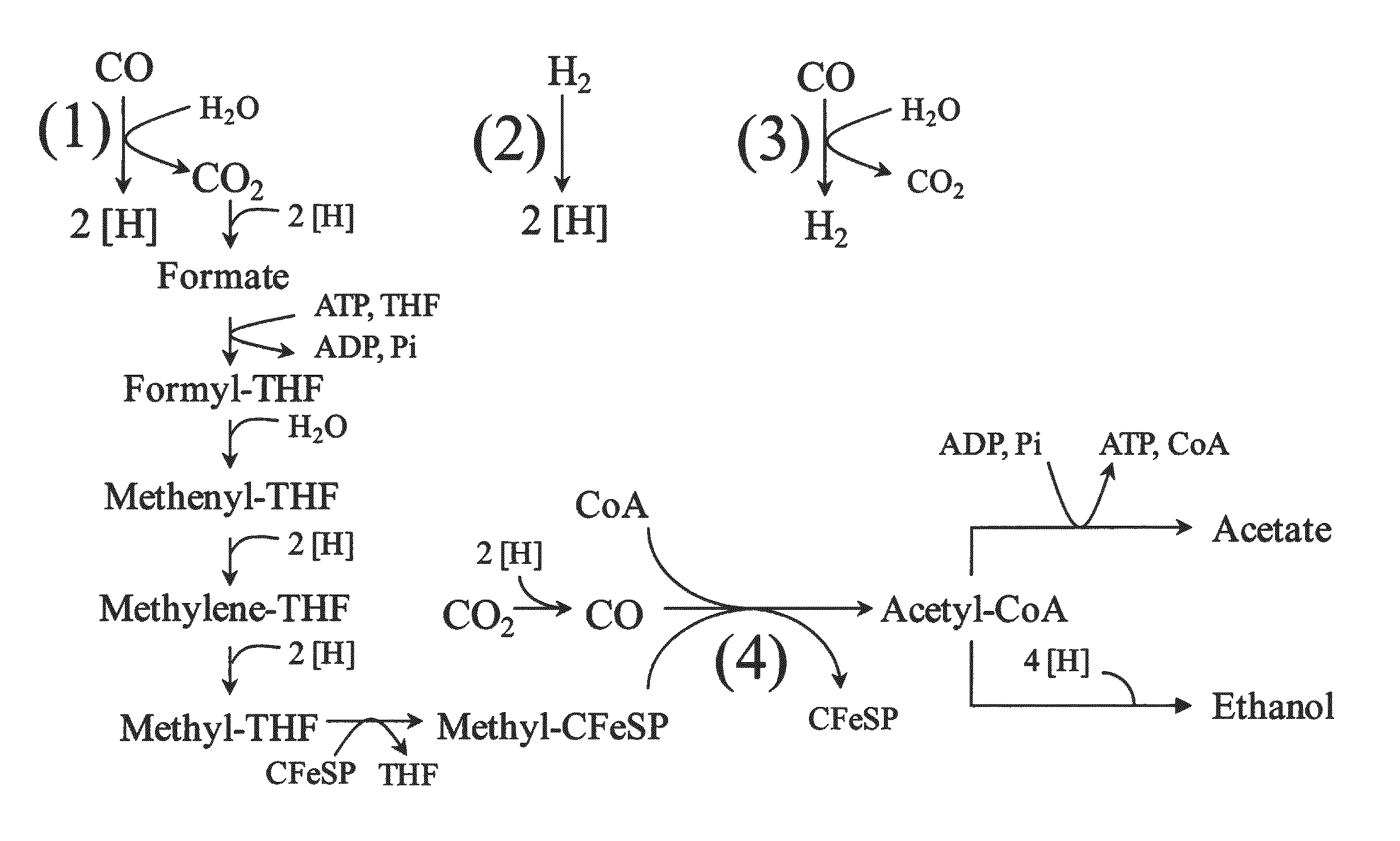
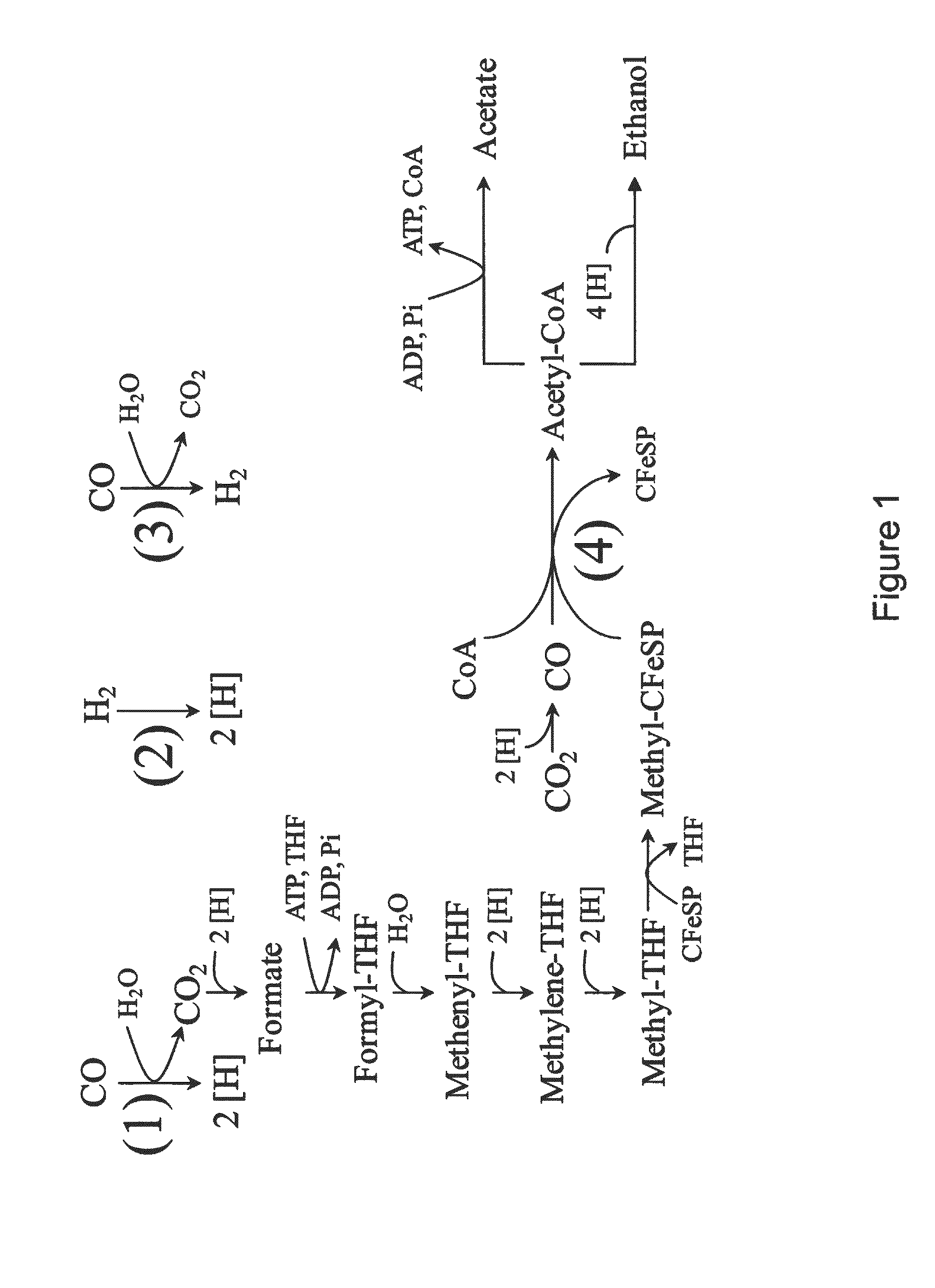
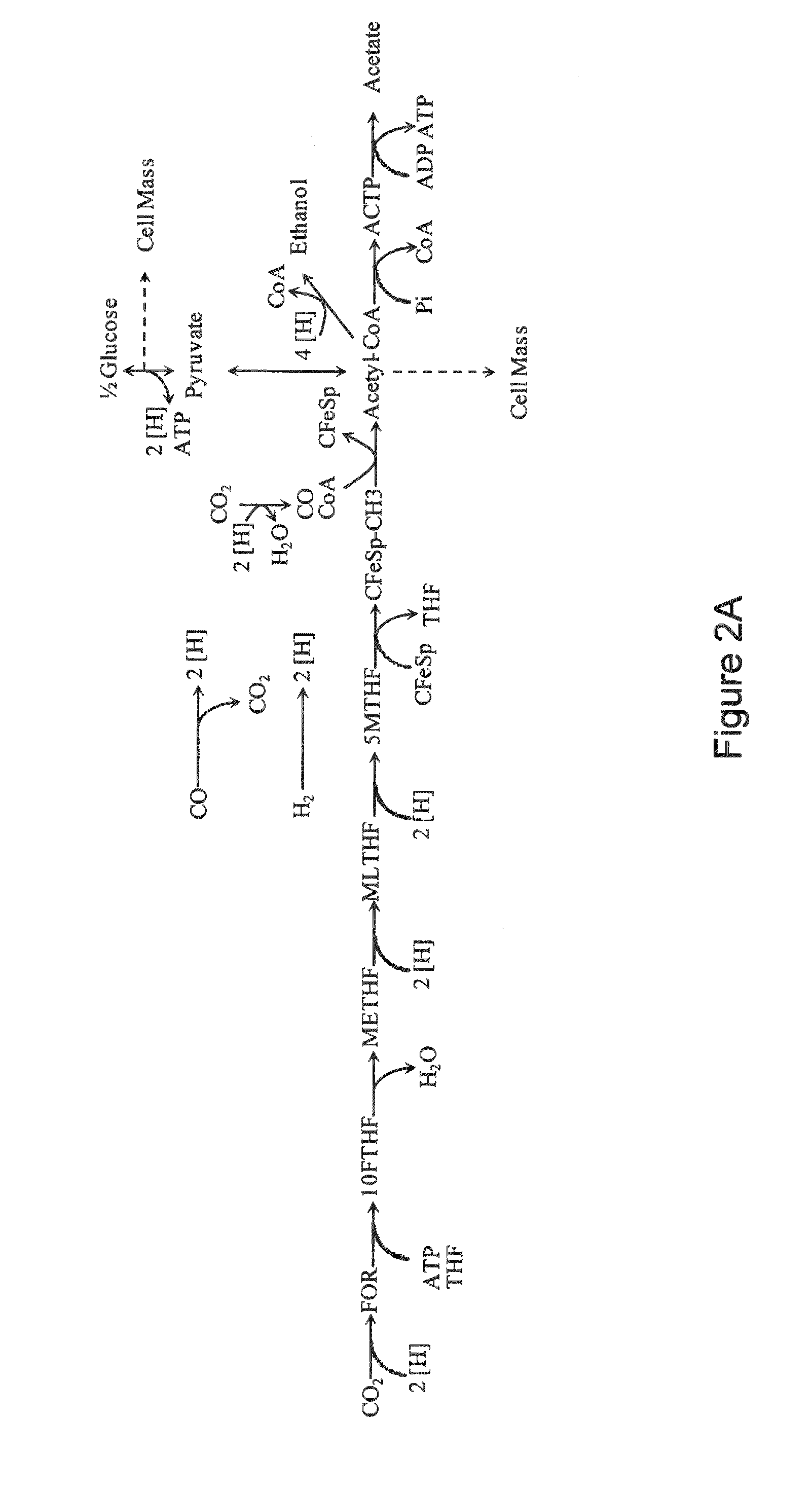
Microorganisms and methods for conversion of syngas and other carbon sources to useful products
A non-naturally occurring microbial organism having an isopropanol, 4-hydroxybutryate, or 1,4-butanediol pathway includes at least one exogenous nucleic acid encoding an isopropanol, 4-hydroxybutryate, or 1,4-butanediol pathway enzyme expressed in a sufficient amount to produce isopropanol, 4-hydroxybutryate, or 1,4-butanediol. The aforementioned organisms are cultured to produce isopropanol, 4-hydroxybutryate, or 1,4-butanediol.
Owner:GENOMATICA INC
Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations
A compound of an angiotensin receptor antagonist (ARB), a neutral endopeptidase inhibitor (NEPi) and one or more monovalent cations are useful for the treatment of hypertension and / or heart failure. ARB includes S—N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine in the anion form, NEPi includes (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester in the anion form and cation includes monovalent cations such as Na+. The compound includes trisodium [3-((1S,3R)-1-biphenyl-4-ylmethyl-3-ethoxycarbonyl-1-butylcarbamoyl)propionate-(S)-3′-methyl-2′-(pentanoyl{2″-(tetrazol-5-ylate)biphenyl-4′-ylmethyl}amino)butyrate] hemipentahydrate.
Owner:NOVARTIS PHARM CORP
Ketone bodies and ketone body esters as blood lipid lowering agents
ActiveUS9211275B2Reduce serum cholesterol and/or triglyceride levelLowering of total serum cholesterol levelHydroxy compound active ingredientsMetabolism disorderChemistryNutritional composition
The subject disclosure provides compositions for reducing serum cholesterol and / or triglyceride levels in subjects. These compositions can comprise racemic β-hydroxybutyrate or D-β-hydroxybutyrate, optionally in the acid form, physiologically compatible salts of racemic β-hydroxybutyrate or D-β-hydroxybutyrate, esters of D-β-hydroxybutyrate, oligomers of D-β-hydroxybutyrate containing from 2 to 20 or more monomeric units in either linear or cyclic form, racemic 1,3 butandiol or R-1,3 butandiol alone and can be, optionally, administered in conjunction with a low fat diet to a subject. Alternatively, compositions comprising racemic β-hydroxybutyrate or D-β-hydroxybutyrate, optionally in the acid form, physiologically compatible salts of racemic β-hydroxybutyrate or D-β-hydroxybutyrate, esters of D-β-hydroxybutyrate, oligomers of D-β-hydroxybutyrate containing from 2 to 20 or more monomeric units in either linear or cyclic form, racemic 1,3 butandiol, R-1,3 butandiol or combinations thereof can be formulated as nutritional supplements (also referred to as nutritional compositions) or incorporated into therapeutic compositions containing a) anti-hypertensive agents; b) anti-inflammatory agents; c) glucose lowering agents; or d) anti-lipemic agents) which are administered to a subject, optionally in combination with a low fat diet, in order to cause a reduction or lowering of: serum cholesterol levels; triglyceride levels; serum glucose levels, serum homocysteine levels, inflammatory proteins (e.g., C reactive protein) and / or hypertension in treated subjects. Alternatively, compositions disclosed herein can be administered alone, or in combination with other therapeutic agents to prevent or reverse vascular disease.
Owner:OXFORD UNIV INNOVATION LTD +1
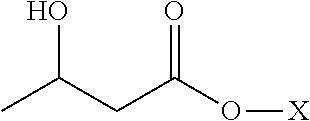
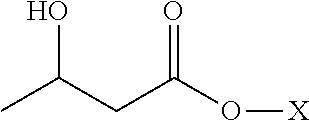
Method for producing optically active 2-(n-substituted aminomethyl)-3-hydroxybutyric acid ester
The present invention relates to a method for producing optically active 2-(N-substituted aminomethyl)-3-hydroxybutyric acid esters wherein a 2-(N-substituted aminomethyl)-3-oxobutyric acid ester is treated with an enzyme source capable of stereoselectively reducing said ester to the corresponding optically active 2-(N-substituted aminomethyl)-3-hydroxybutyric acid ester having the (2S,3R) configuration. The present invention provides an efficient method for industrially producing optically active 2-(N-substituted aminomethyl)-3-hydroxybutyric acid esters, in particular such compounds having the (2S,3R) configuration, which are useful as intermediates for the production of medicinal compounds, among others.
Owner:KANEKA CORP
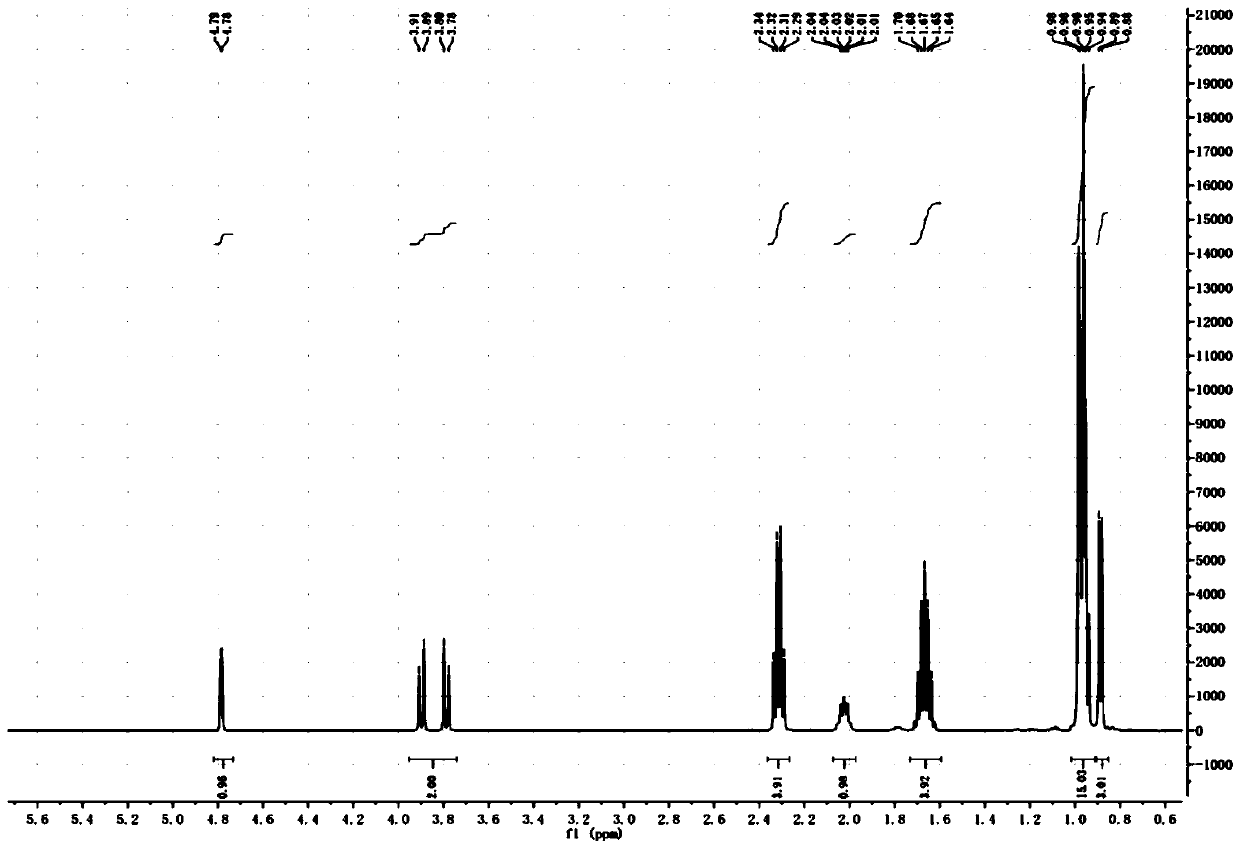
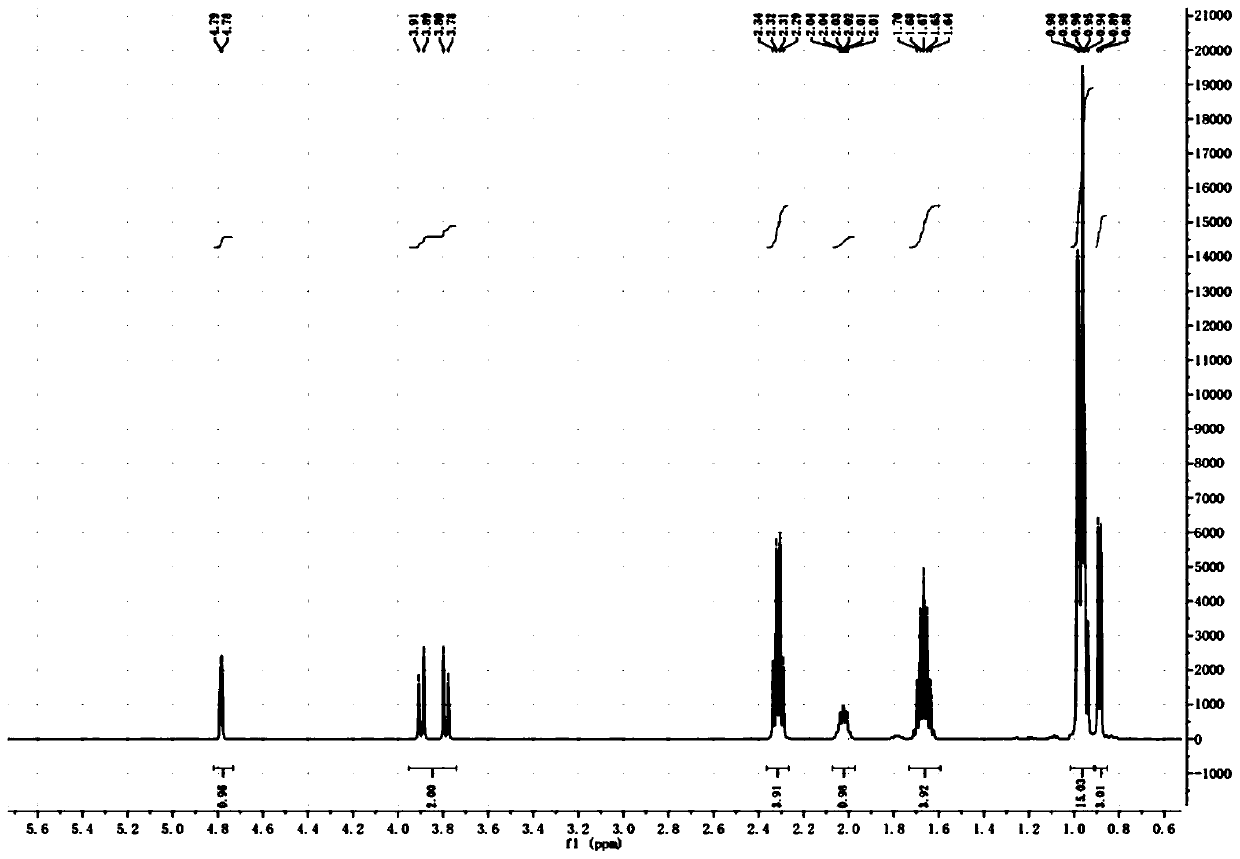
SYNTHETIC METHODS FOR PREPARATION OF (S)-(2R,3R,11bR)-3-ISOBUTYL-9,10-DIMETHOXY-2,3,4,6,7,11b-HEXAHYDRO-1H-PYRIDO[2,1-a]ISOQUINOLIN-2-YL 2-AMINO-3-METHYLBUTANOATE DI(4-METHYLBENZENESULFONATE)
ActiveUS20170183346A1Safe and efficient and cost-effective and readily scalableSafe and efficient and and readily methodOrganic chemistryPyridine3-Methylbutanoic acid
Provided herein are processes for the preparation of (S)-(2R,3R,11bR-3-isobuty-9,10-dimethoxy-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinolin-2-yl 2-amino-3-methylbutanoate di(4-methylbenzenesulfonate), or a solvate, hydrate, or polymorph thereof.
Owner:NEUROCRINE BIOSCI INC
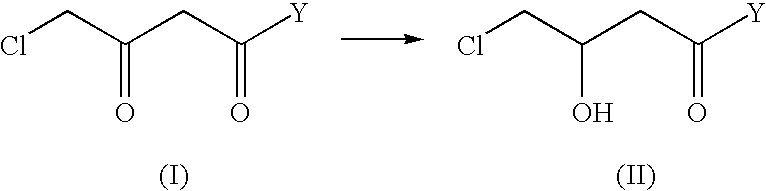
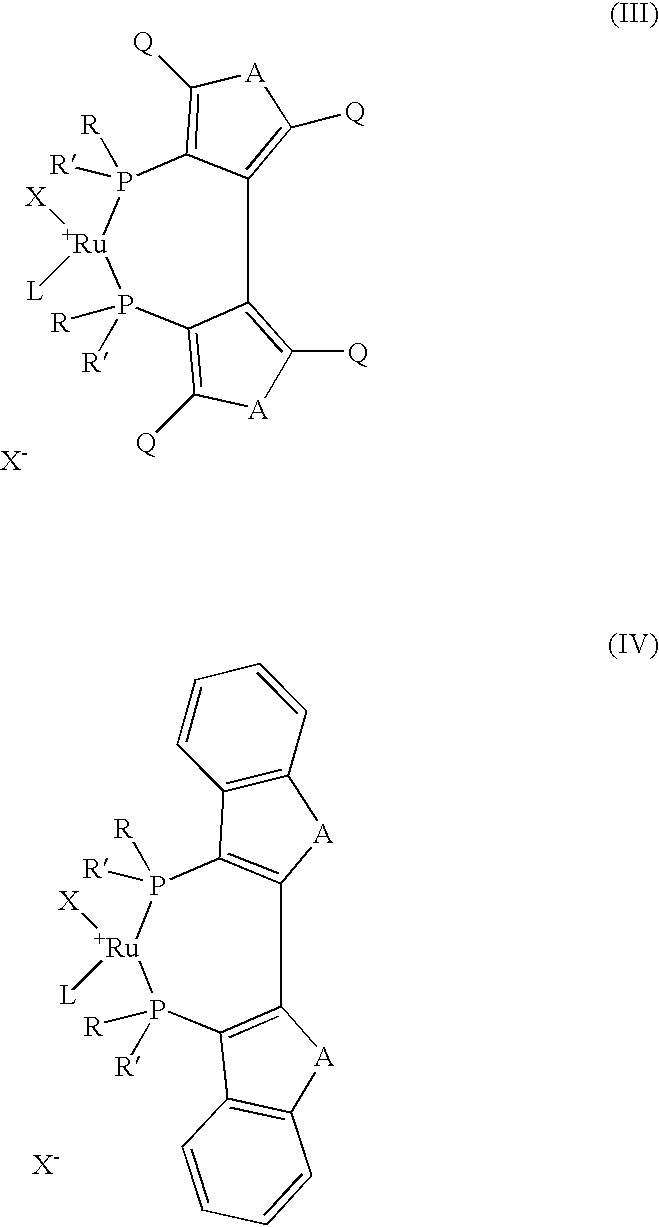
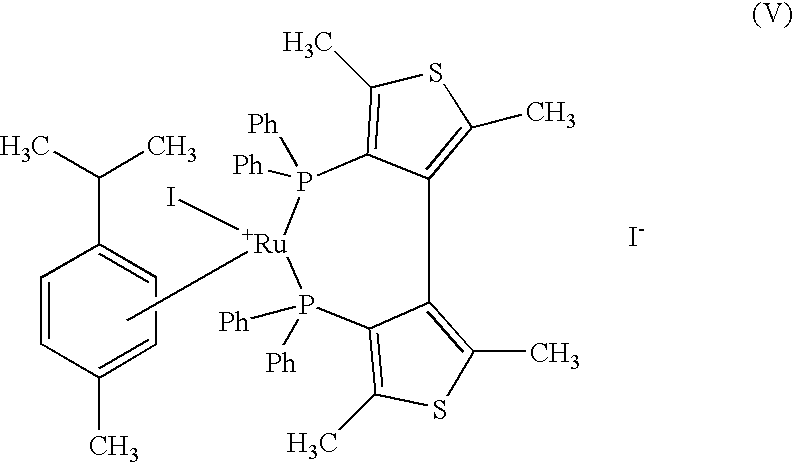
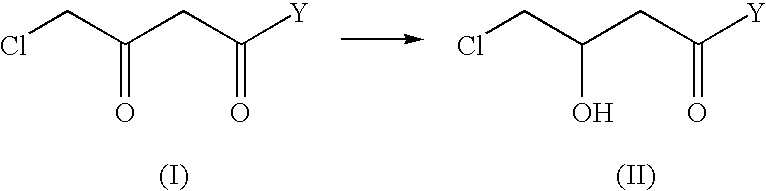
Industrial process for the production of L-carnitine
InactiveUS6566552B2Easily appilcable on an industrial scaleReduce productionOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydrogen pressureSubstrate concentration
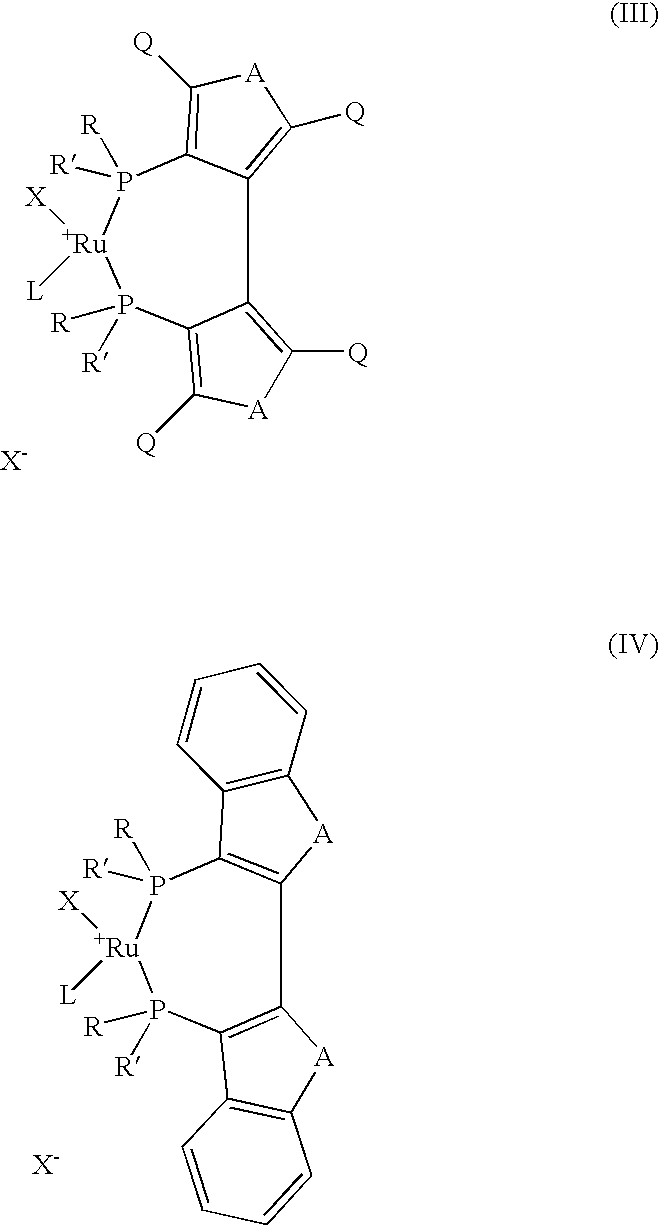
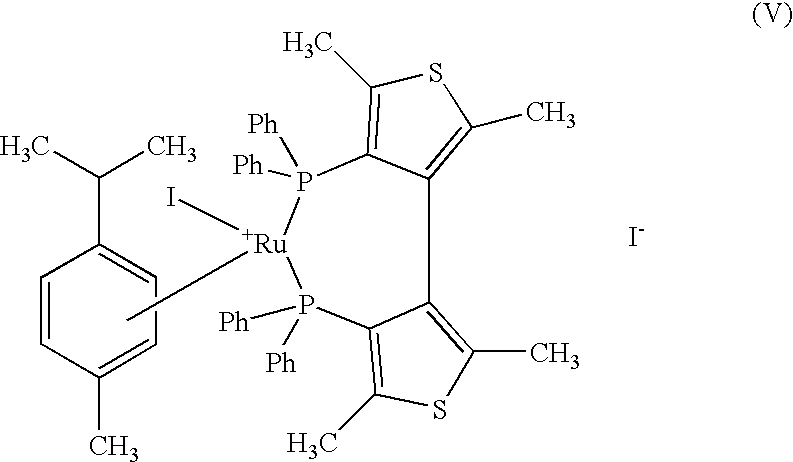
The present invention describes a process for the industrial production of L-carnitine, comprising the enantioselective reduction of an alkyl 4-chloro-3-oxobutyrate or 4-chloro-3-oxobutyramide. The optically active 3-hydroxy derivative thus obtained is reacted with trimethylamine, obtaining crude L-carnitine, which is then finally purified. The catalyst used for the reduction is a complex of ruthenium bound to a penta-atomic bis-heteroaromatic system. The reduction reaction, performed in controlled conditions of hydrogen pressure, substrate concentration, temperature, and substrate: catalyst molar ratio, enables 4-chloro-3-hydoxybutyrate or 4-chloro-hydroxybutyamide to be obtained in a high yield. The process described, which leads to L-carnitine being obtained, is easily applicable on an industrial scale.
Owner:SIGMA TAU IND FARMACEUTICHE RIUNITE SPA
Industrial process for the production of L-carnitine
InactiveUS20020165408A1Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydrogen pressureEthyl Chloride
The present invention describes a process for the industrial production of L-carnitine, comprising the enantioselective reduction of an alkyl 4-chloro-3-oxobutyrate or 4-chloro-3-oxobutyramide. The optically active 3-hydroxy derivative thus obtained is reacted with trimethylamine, obtaining crude L-carnitine, which is then finally purified. The catalyst used for the reduction is a complex of ruthenium bound to a penta-atomic bis-heteroaromatic system. The reduction reaction, performed in controlled conditions of hydrogen pressure, substrate concentration, temperature, and substrate: catalyst molar ratio, enables 4-chloro-3-hydoxybutyrate or 4-chloro-hydroxybutyamide to be obtained in a high yield. The process described, which leads to L-carnitine being obtained, is easily applicable on an industrial scale.
Owner:SIGMA TAU IND FARMACEUTICHE RIUNITE SPA
Non-racemic beta-hydroxybutyrate compounds and compositions enriched with the R-enantiomer and methods of use
ActiveUS10596131B2Great and faster effectImprove the level ofHydroxy compound active ingredientsPharmaceutical delivery mechanismButyrateChemical compound
Ketogenic compositions including a non-racemic mixture of beta-hydroxybutyrate (BHB) enriched with the R-enantiomer are formulated to increase ketone body level in a subject. The non-racemic mixture of BHB is enriched with the R-enantiomer to elevate ketone bodies and increase the rate at which ketosis is achieved yet contains an amount of the S-enantiomer sufficient to provide alternative benefits as discussed herein. In some aspects a composition for increasing ketone body level in a subject contains a dietetically or pharmaceutically acceptable carrier and a non-racemic mixture of R-beta-hydroxybutyrate and S-beta-hydroxybutyrate, wherein the non-racemic mixture of R-beta-hydroxybutyrate and S-beta-hydroxybutyrate contains from about 51% to 99.5% by enantiomeric equivalents of the R-beta-hydroxybutyrate and from about 49% to about 0.5% by enantiomeric equivalents of S-beta-hydroxybutyrate enantiomer.
Owner:AXCESS GLOBAL SCI LLC
Enzymatic process for obtaining 17 alpha-monoesters of cortexolone and/or its 9,11-dehydroderivatives
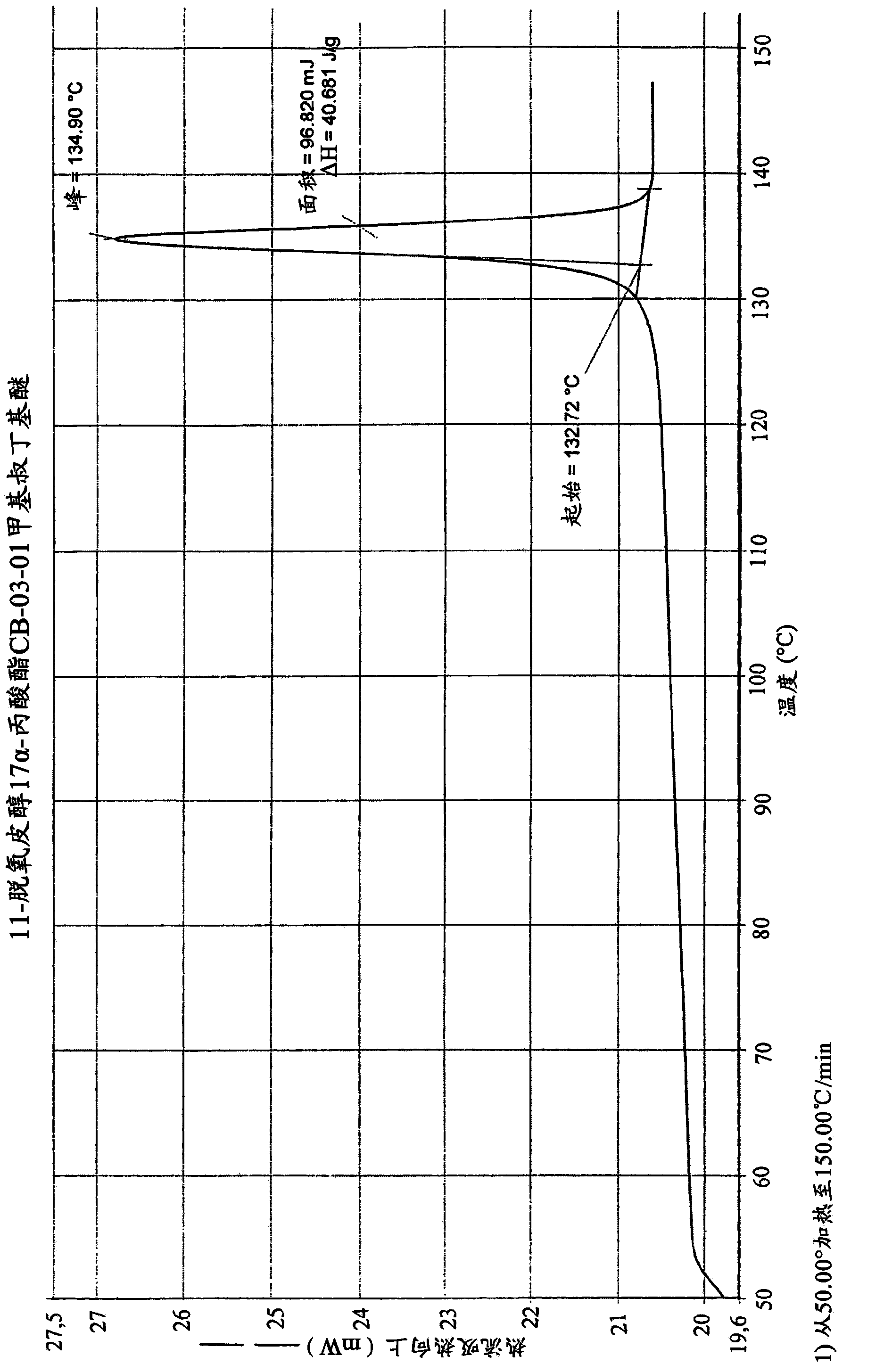
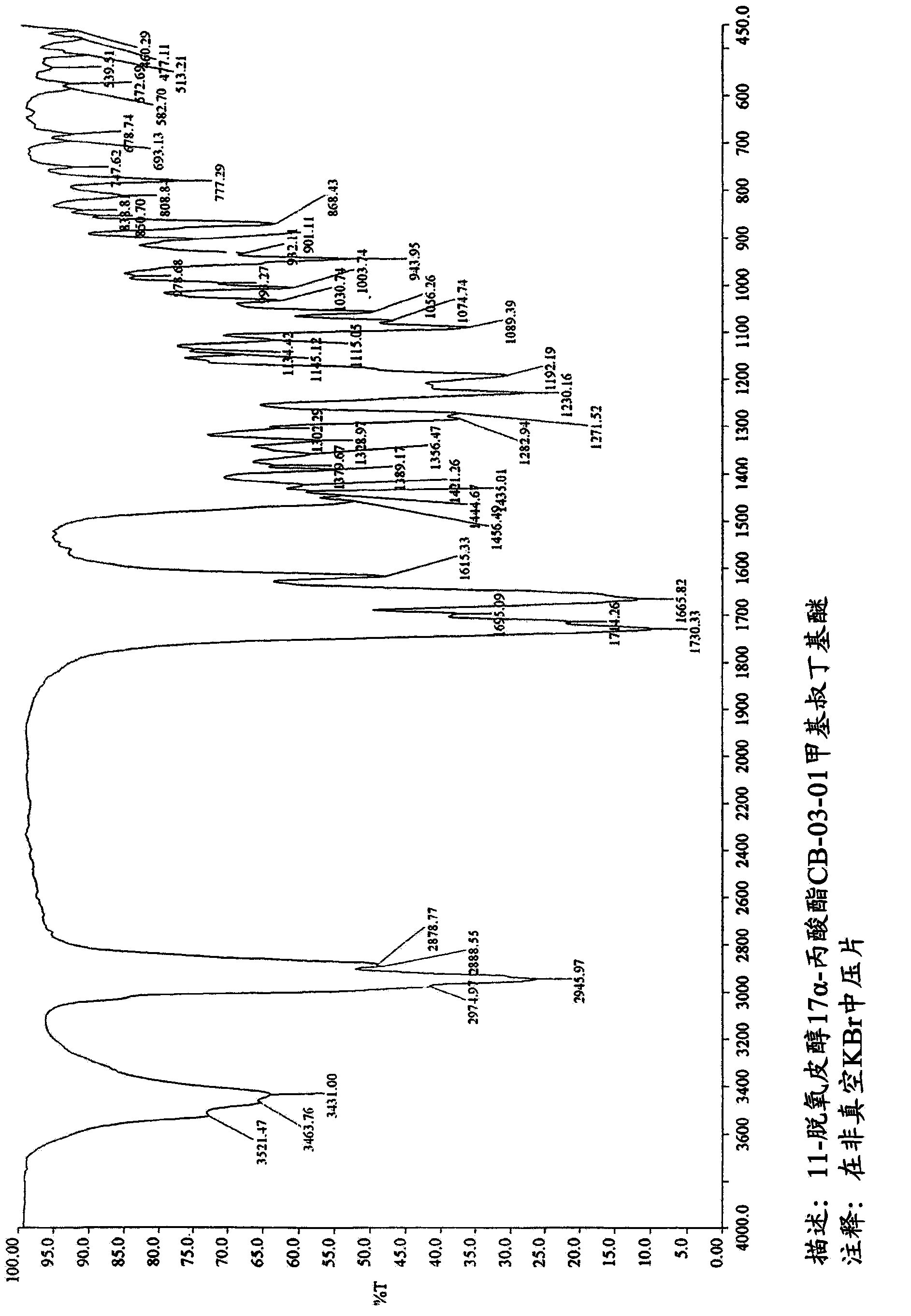
The present invention refers to a new enzymatic process for obtaining 17a- monoesters of cortexolone and / or its 9,11-dehydroderivatives starting from the corresponding 17alpha,21-diesters which comprises an enzymatic alcoholysis reaction. Furthermore, the present invention refers to new crystalline forms of cortexolone 17alpha-propionate and 9,11-dehydro-cortexolone 17alpha-butanoate.
Owner:CASSIOPEA SPA
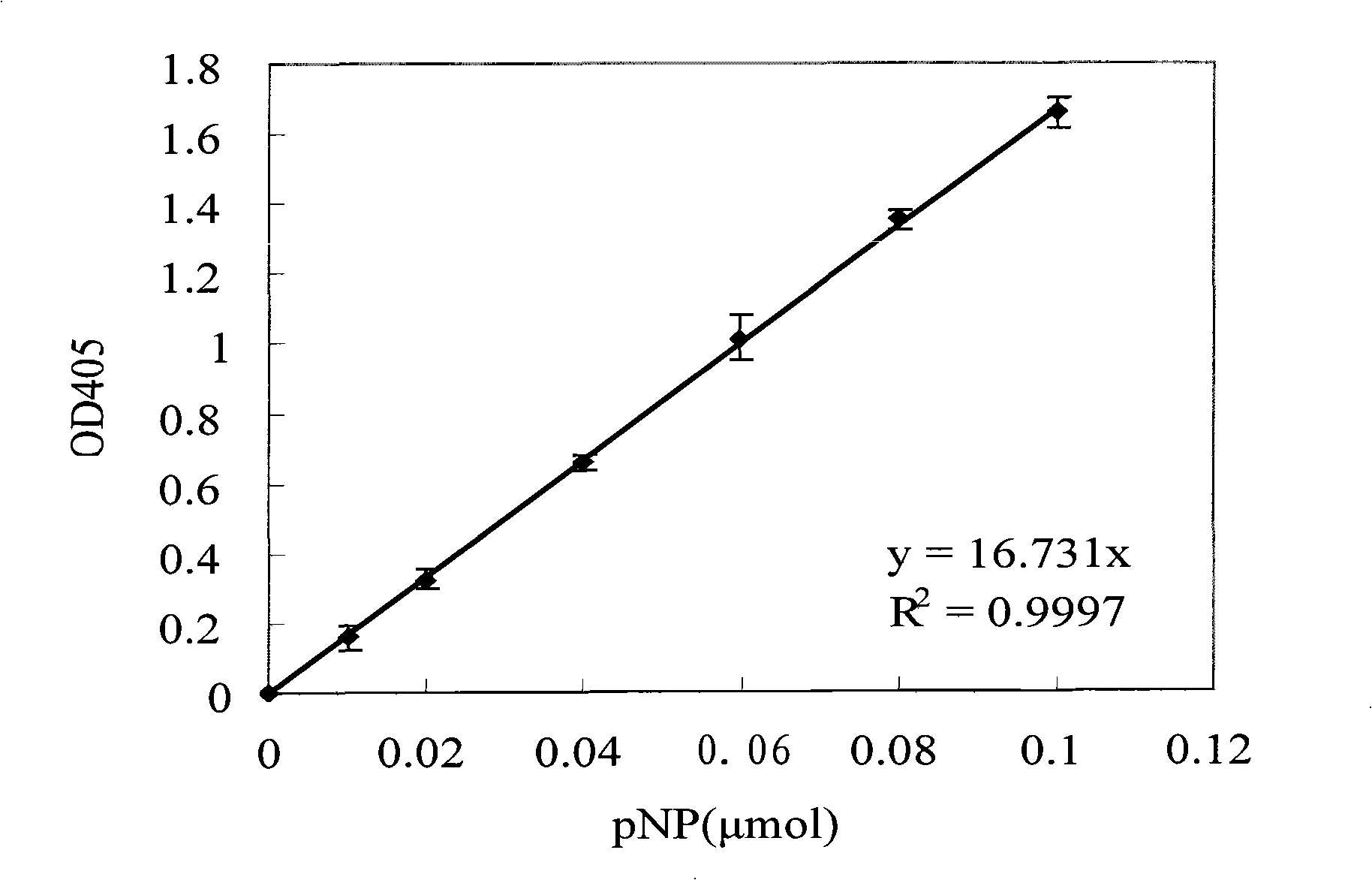
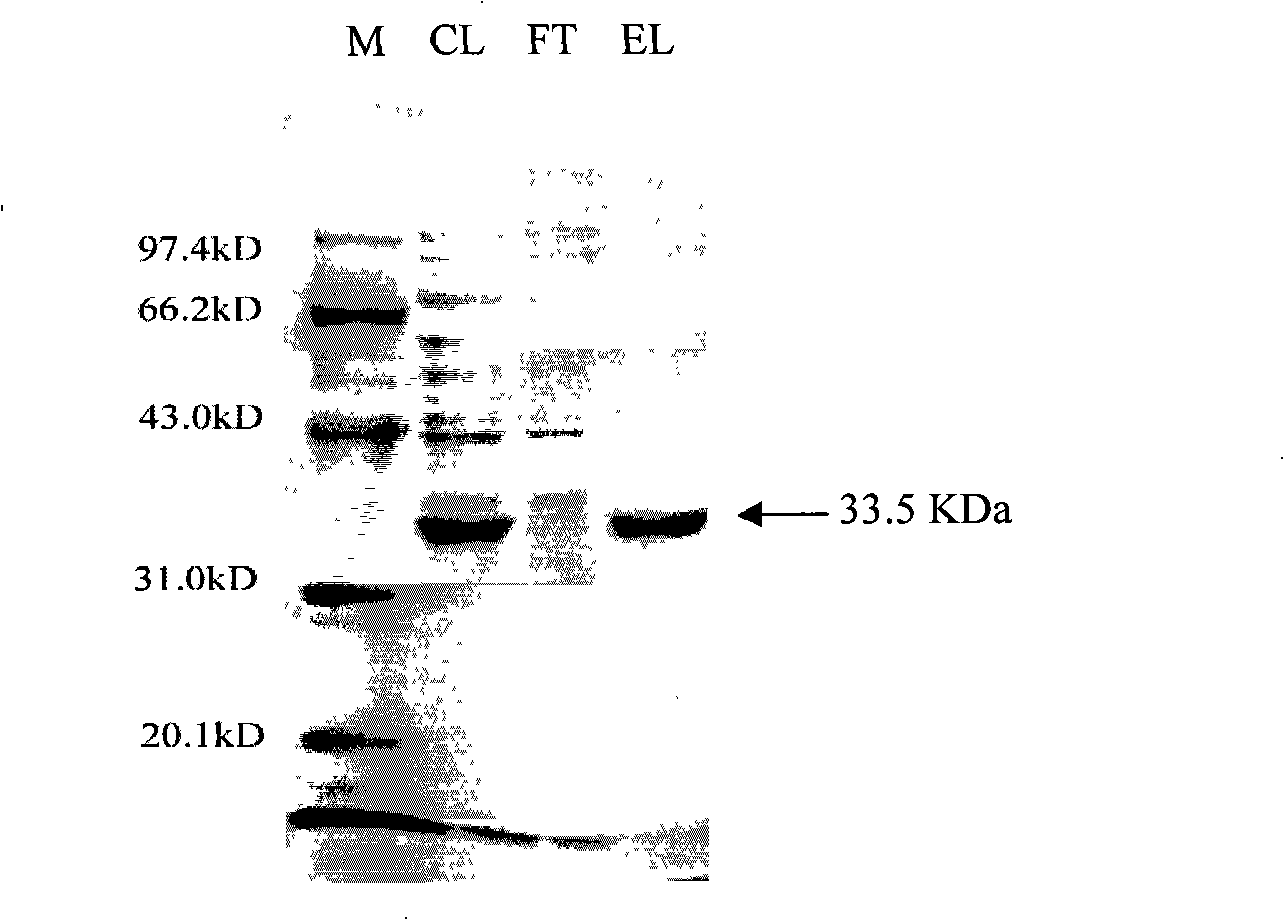
Novel gene of esterase and recombinant expression system
The invention provides a new esterase gene named Est_p1 and a recombination expression system thereof. The gene is cloned from the metagenomic library of the marginal mud 100 meters under the South China Sea. The gene is an 891bp long amino acid numbered 296, which successfully constructs a recombination expression system of a colon bacillus named pET28a-Est_p1 / BL21. The molecular weight of a target protein is 33.5 kD. With pNP-butyric ester as catalyzing substrates, the Est_p1 is proved to be a mildly alkaline medium temperature esterase. The Est_p1 has strong catalytic activity for short esters and is applicable to esterification and transesterification industries.
Owner:CHINA AGRI UNIV
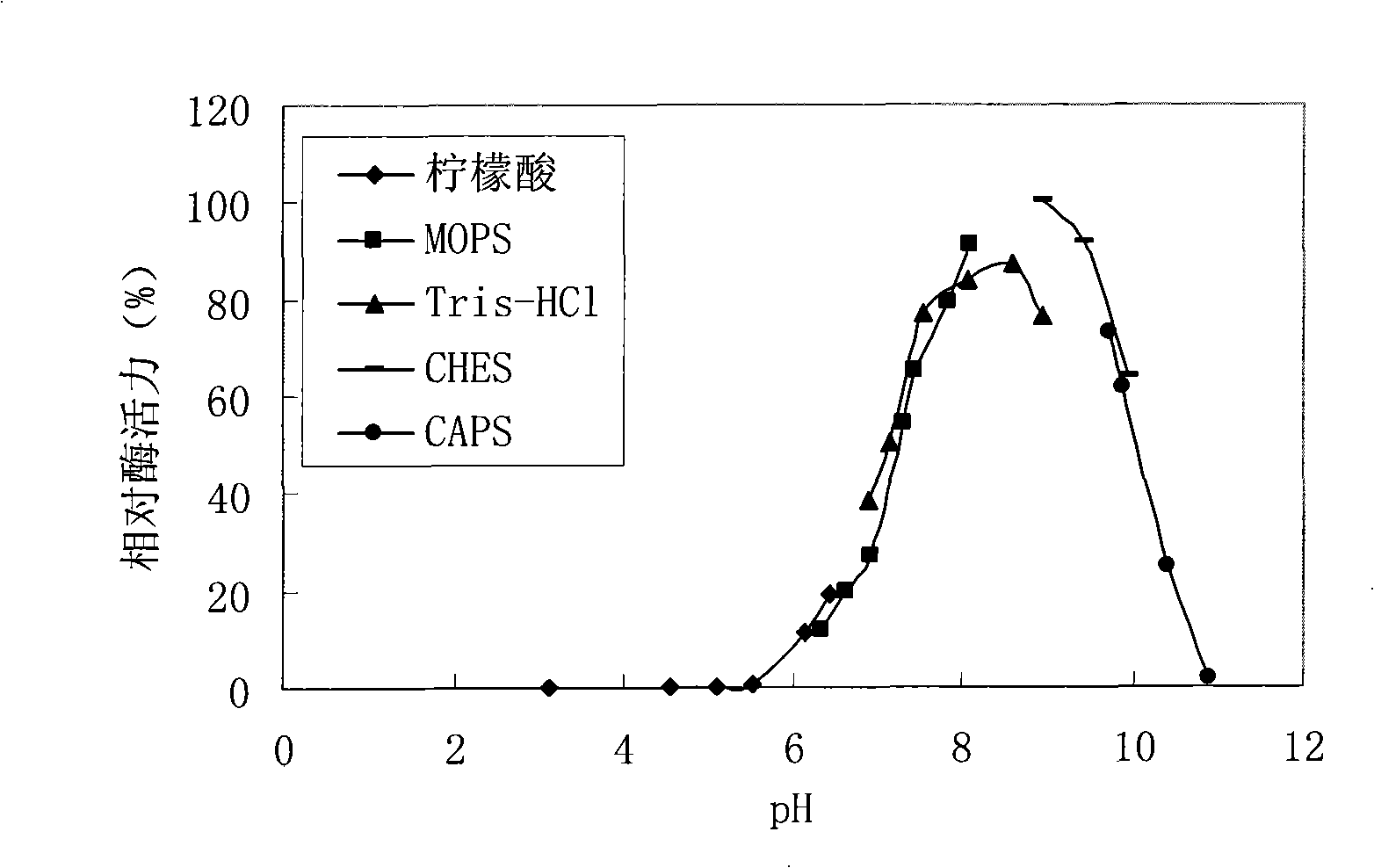
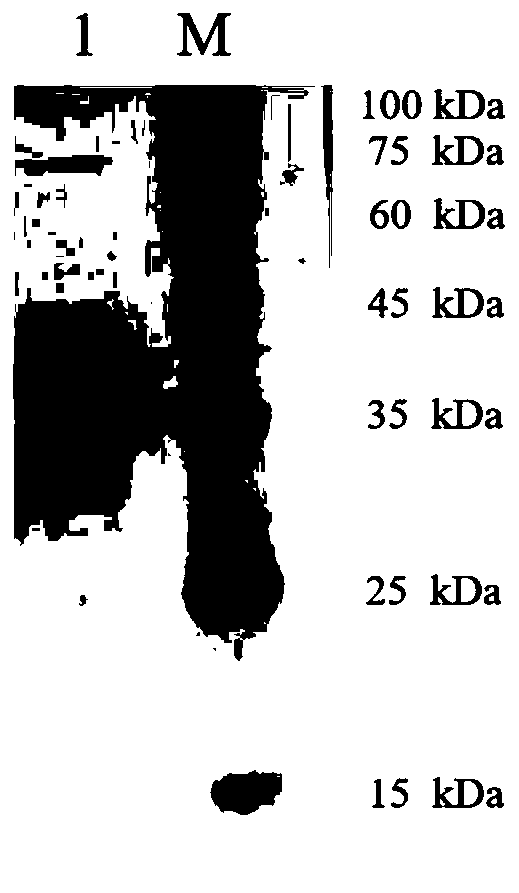
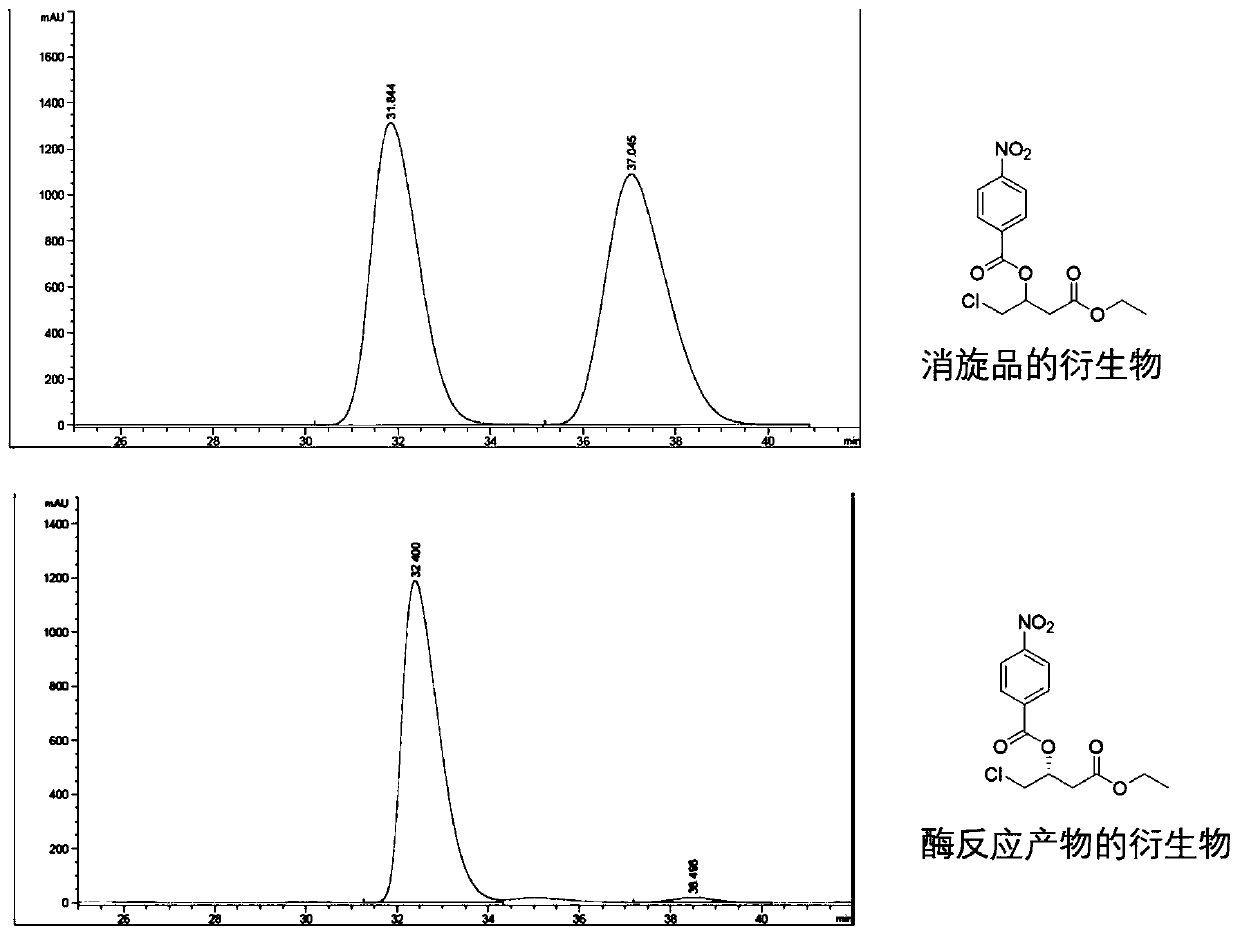
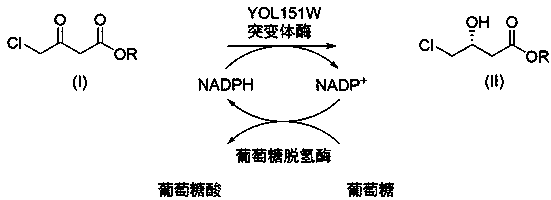
Carbonyl reductase mutant and application thereof in preparation of (R)-4-chloro-3-hydroxy-butyrate
ActiveCN111172124AHigh substrate concentrationHigh stereoselectivityBacteriaOxidoreductasesCarbonyl ReductaseBio engineering
The invention belongs to the technical fields of biological pharmacy and biological engineering, and particularly relates to a carbonyl reductase mutant and application thereof in preparation of (R)-4-chloro-3-hydroxy-butyrate. The carbonyl reductase mutant disclosed by the invention is obtained by replacing phenylalanine at the 85th site of a sequence shown as SEQ ID NO.2 with methionine, or is obtained by replacing one or more sites except the 85th site with amino acid residues on the basis that phenylalanine at the 85th site of the sequence shown as SEQ ID NO.2 is replaced with methionine.The invention also relates to a recombinant expression vector containing the carbonyl reductase mutant gene, an engineering bacterium containing the carbonyl reductase mutant gene and a glucose dehydrogenase gene, and application of the genetic engineering bacterium in preparation of (R)-4-chloro-3-hydroxy-butyrate by asymmetric reduction of chloroacetoacetate. Compared with a wild enzyme, the carbonyl reductase mutant disclosed by the invention has the advantages that the reducing capability on chloroacetoacetate is obviously improved and the carbonyl reductase mutant has good industrial application prospect.
Owner:FUDAN UNIV
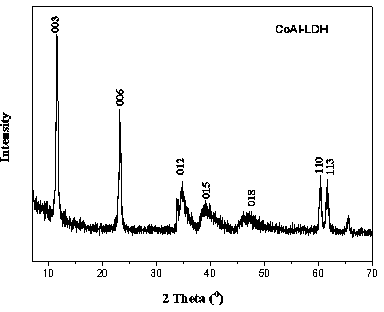
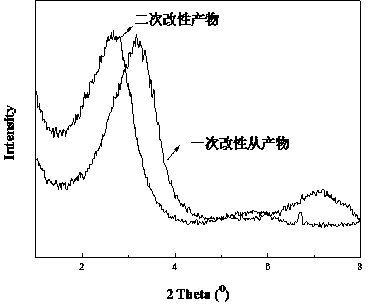
Preparation method and application of secondary modified layered double hydroxide
InactiveCN103965661AIncrease layer spacingImprove thermal stabilityPigment treatment with non-polymer organic compoundsPolyesterButyrate
The invention relates to a preparation method and application of secondary modified layered double hydroxide. The preparation method comprises the following steps: performing primary modification on layered double hydroxide by using lauric acid anions, and performing secondary modification on the layered double hydroxide subjected to the primary modification by using aliphatic cationic compounds such as cetyl trimethylammonium bromide and the like. Compared with the layered double hydroxide usually subjected to only one modification process in the prior art, the secondary modified double hydroxide subjected to two modification processes has larger interlayer spacing and higher heat stability, and can be used as reinforcer in a biological polyester poly(3-hydroxybutyrate-co-4-hydroxybutyrate) material.
Owner:NANJING FORESTRY UNIV
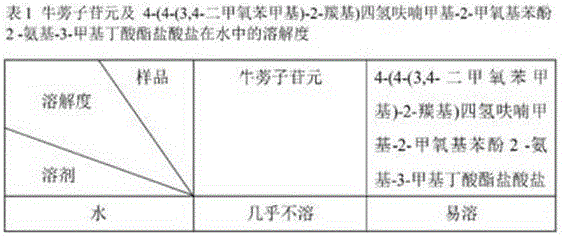
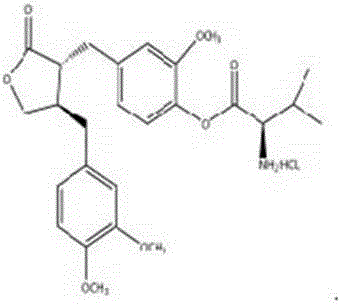
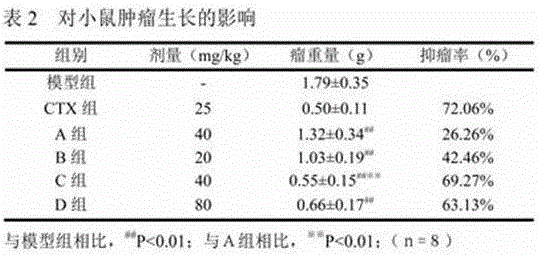
Application of arctigenin valine ester hydrochloride to preparation of anti-tumor drugs
The invention provides a 4-(4-(3, 4-dimethoxy benzene methyl)-2-carbonyl) tetrahydrofurfuryl-2-methoxyphenol 2-amino-3-methyl butyric acid ester hydrochloride for resisting tumors, and belongs to the field of research of anti-tumor drugs. Experimental results indicate that the 4-(4-(3, 4-dimethoxy benzene methyl)-2-carbonyl) tetrahydrofurfuryl-2-methoxyphenol 2-amino-3-methyl butyric acid ester hydrochloride has obvious inhibition effect on hepatocarcinoma 22 solid tumor-bearing mice, immune organs of organisms can be protected to some extent, and immune functions of the organisms are enhanced.
Owner:JILIN AGRICULTURAL UNIV
Polymethyl polyglycol methacrylate containing paracetamol structure as well as preparation method and use method thereof
InactiveCN102344534AWith analgesic functionExcellent anticoagulant propertiesPharmaceutical containersMedical packagingReaction temperatureNitrogen gas
The invention discloses a polymethyl polyglycol methacrylate containing a paracetamol structure as well as a preparation method and a use method thereof. The preparation method comprises the following steps of: mixing 4-nitrate ester-pacrylamide phenyl butyrate compounds, methyl acrylate compounds and methylpropenoic polyglycol acid ester compounds; polymerizing free groups under the protection of nitrogen gas; reacting at the temperature of 20-90DEG C for 1-20 hours; repeatedly precipitating and dissolving generated polymerisate by petroleum ether and tetrahydrofuran; and finally, vacuum drying. The obtained polymer has a certain analgesic function and a better anticoagulation effect.
Owner:TIANJIN POLYTECHNIC UNIV
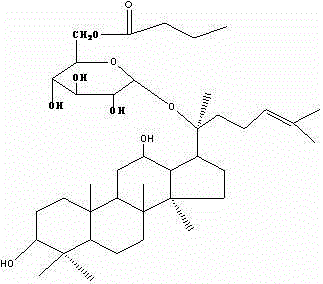
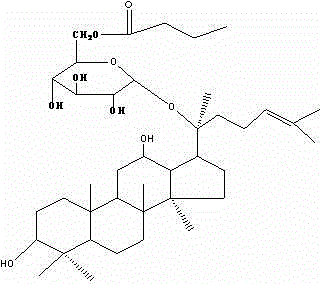
Preparation of ginsenoside M1 n-butyrate and application of antidiabetic medicines of ginsenoside M1 n-butyrate
InactiveCN102942610ASuitable for industrial productionIncrease productionOrganic active ingredientsMetabolism disorderDiabetes mellitusButyrate
The invention provides ginsenoside M1 n-butyrate which is a new compound with a molecular formula of C40H69O9. The molecular weight of the ginsenoside M1 n-butyrate is 692. The invention further discloses a preparation method of the compound and application of antidiabetic medicines of the compound. The ginsenoside M1 n-butyrate can resist to physiological activity of diabetes mellitus and can be used for preventing and treating the diabetes mellitus and conducting assistant treating the diabetes mellitus.
Owner:JILIN AGRICULTURAL UNIV
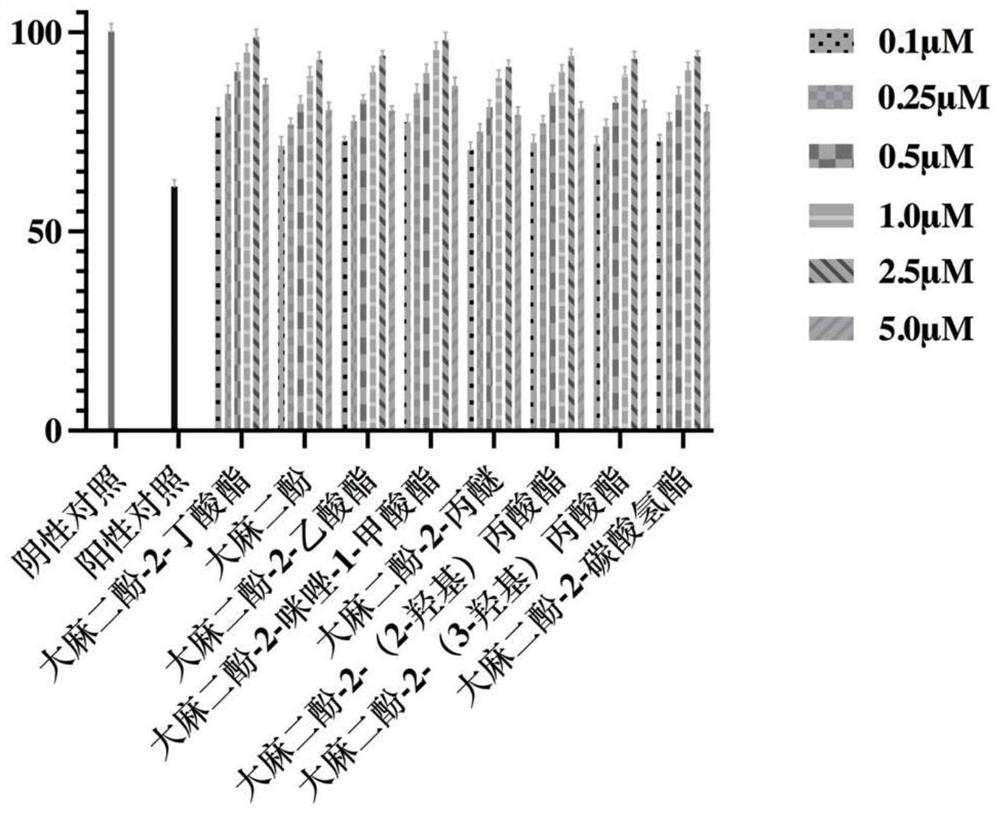
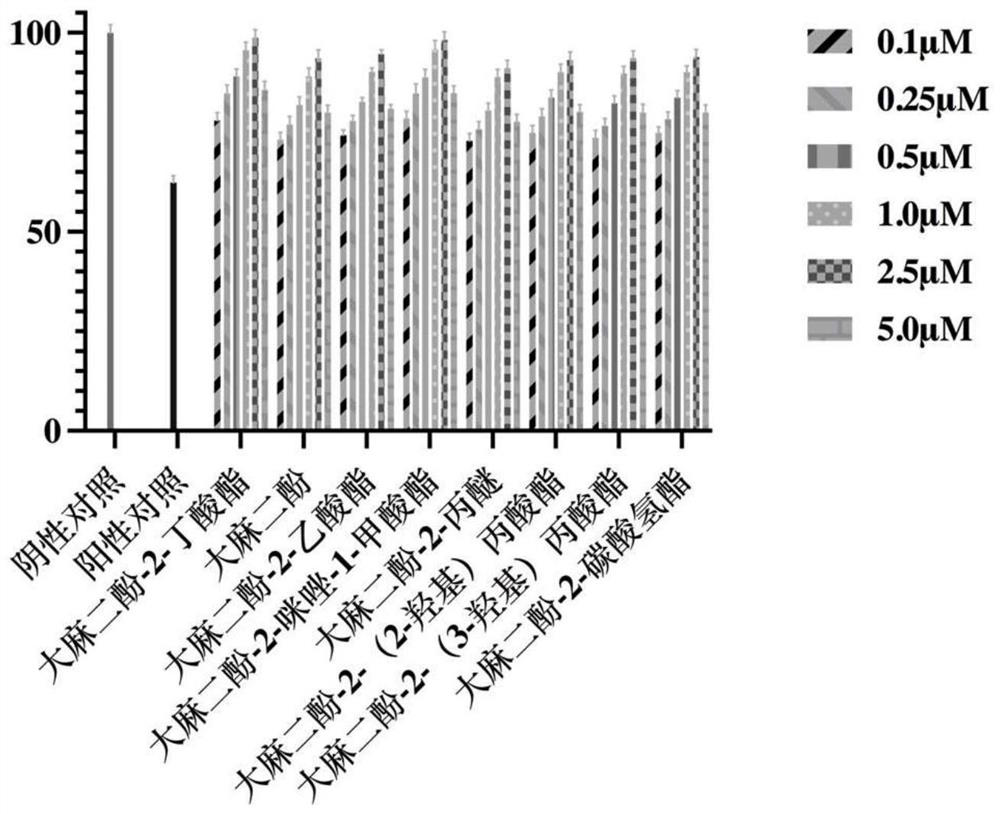
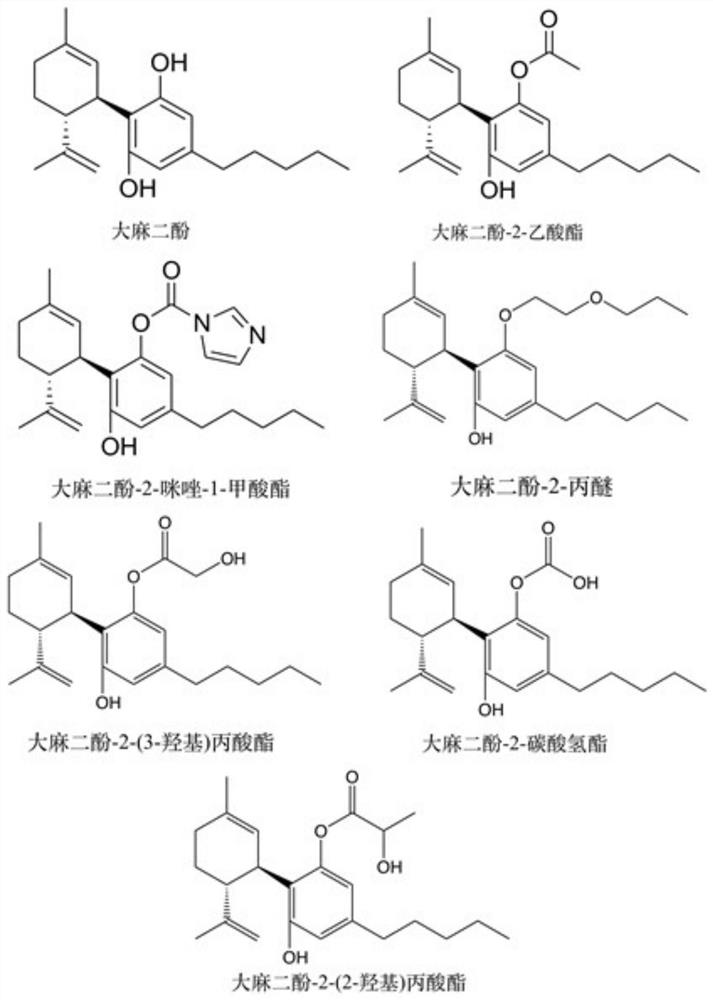
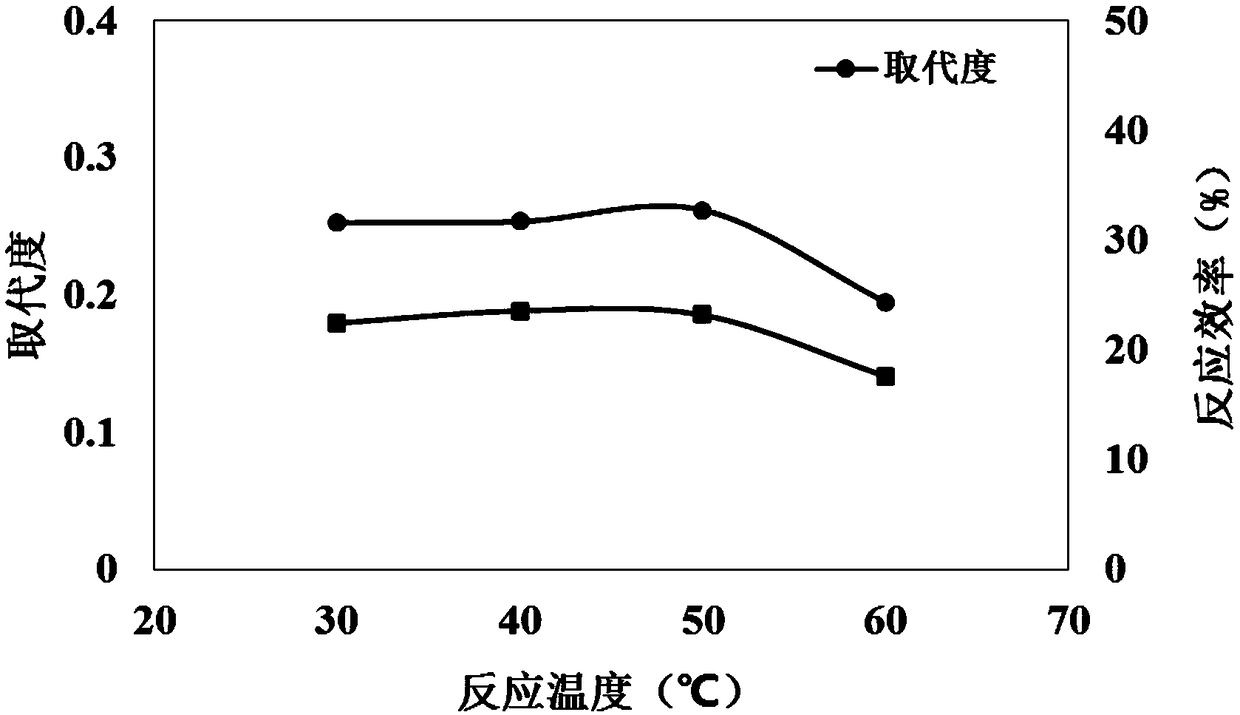
Cannabidiol 2-butyrate and application thereof
ActiveCN113735709AProtectiveRaw materials are cheap and easy to getCosmetic preparationsNervous disorderButyrateApoptosis
The invention discloses cannabidiol 2-butyrate and application thereof, and particularly relates to a compound as shown in a formula I, and a stereoisomer or pharmaceutically acceptable salt thereof. The cannabidiol 2-butyrate has a protection effect on nerve cell injury and skin cell injury, and can induce apoptosis of human breast cancer cells. The compound has practical application value in medicine and cosmetic production.
Owner:黑龙江丰佑麻类种植有限公司
Butyrate starch preparing method
ActiveCN109400725ASolve the problem of irritating odor and volatilePrevent volatilizationFood ingredient functionsButyric anhydrideLarge intestine
The invention discloses a butyrate starch preparing method and belongs to the technical fields of preparation and application of modified starch and deep processing of agricultural products. Accordingto the butyrate starch preparing method, starch serve as the principal raw material for esterification with butyric anhydride to achieve stabilization of butyric acid and small releasing of the butyric acid in gastric juice; resistant starch prepared through esterification of the butyric acid and the starch can well avoid being absorbed in small intestines, resist digestion by enzymes secreted bybrush borders, selectively release micromolecular effective load in large intestines; fermented by enteric microorganisms, the resistant starch can produce more short-chain fatty acids and probiotics, thereby improving the bioavailability of the butyric acid and expanding the application range of the starch. Compared with other butyric acid products, the butyrate starch is odorless and can be esterified into the resistant starch to convey the butyric acid to the rear end of the intestines; the butyrate starch can be fermented by the enteric microorganisms and decomposed by bacterial esteraseinto butyric acid and other short-chain fatty acids and increase the content of the probiotics, thereby belonging to probiotic products.
Owner:JIANGNAN UNIV
Non-racemic beta-hydroxybutyrate compounds and compositions enriched with the R-enantiomer and methods of use
ActiveUS11103470B2Improve the level ofReduce loadPowder deliveryHydroxy compound active ingredientsHydroxybutyric acidButyrate
Ketogenic compositions include a non-racemic mixture of beta-hydroxybutyrate salts and acid(s) enriched with the R-enantiomer. The compositions are enriched with the R-enantiomer to elevate ketone bodies and increase the rate at which ketosis is achieved yet contains an amount of the S-enantiomer to provide alternative benefits. Beta-hydroxybutyric acid is more rapidly absorbed and utilized by the body than salts or esters, enhances taste, and reduces the need to include citric acid or other edible acids. Beta-hydroxybutyrate salts are more slowly absorbed and utilized by the body and can provide one or more electrolytes. Compositions for increasing ketone body level in a subject may contain a dietetically or pharmaceutically acceptable carrier and a non-racemic mixture of R-beta-hydroxybutyrate and S-beta-hydroxybutyrate, wherein the non-racemic mixture of R-beta-hydroxybutyrate and S-beta-hydroxybutyrate contains from about 50.5% to 99.5% by enantiomeric equivalents of R-beta-hydroxybutyrate and from about 49.5% to about 0.5% by enantiomeric equivalents of S-beta-hydroxybutyrate.
Owner:AXCESS GLOBAL SCI LLC
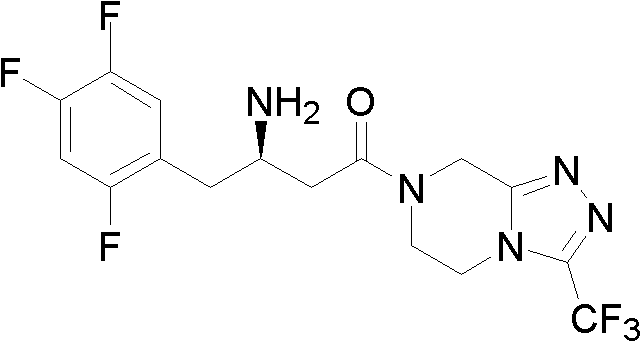
Preparation methods for sitagliptin intermediates
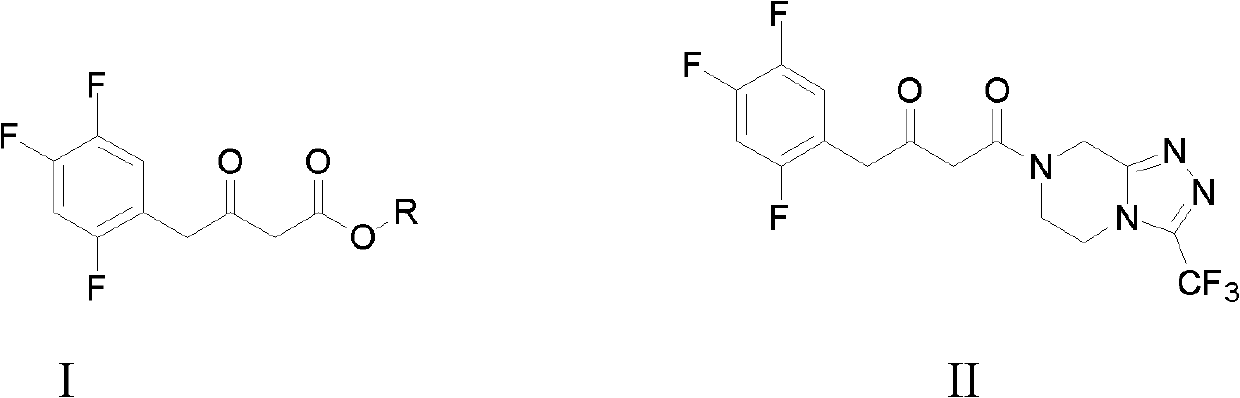
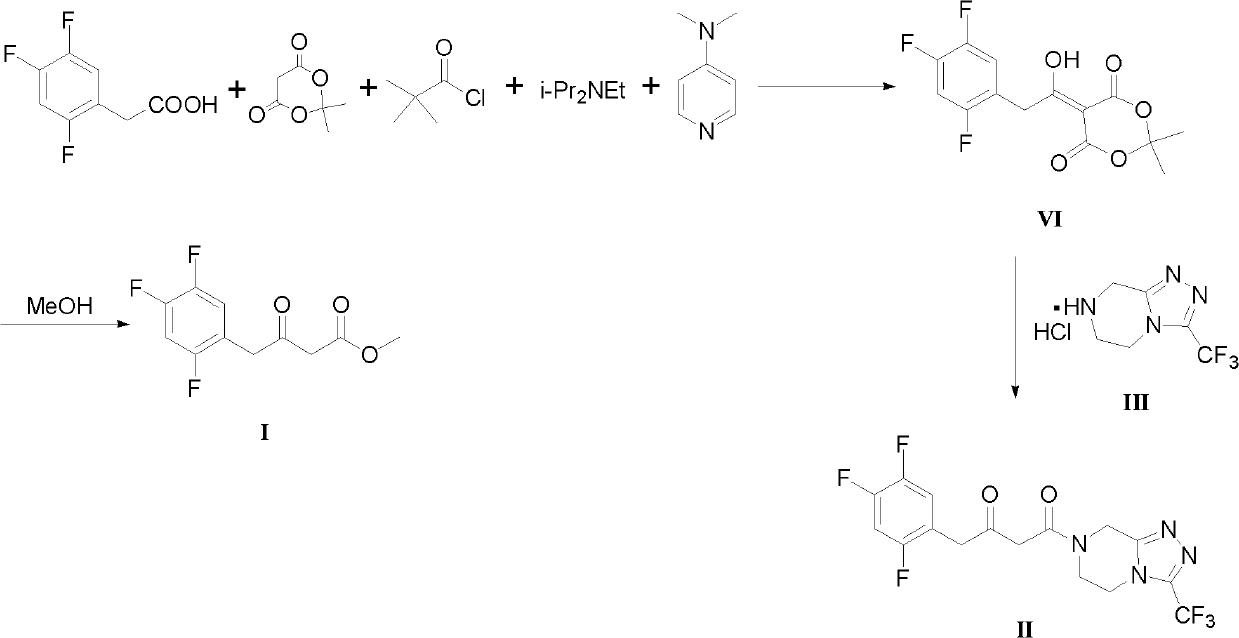
ActiveCN102503829AMild reaction conditionsSimple and fast operationOrganic compound preparationCarboxylic acid esters preparationGrignard reagentPyrazine
The invention discloses preparation methods for sitagliptin intermediates including 3-carbonyl-4-(2,4,5-trifluorophenyl)-butyrate (I) and 1-(3-trifluoromethyl-5,6-dihydro-8H-(1,2,4) triazole-(4,3-a) pyrazine-7-)-4-(2,4,5-trifluorophenyl)-1,3-butanedione (II). The preparation method for the intermediate (I) includes the steps of preparing corresponding Grignard reagent by bromoacetate and magnesium powder by means of Grignard reaction, and preparing the intermediate I by means of addition reaction between the Grignard reagent and 2,4,5-trifluoro-benzeneacetonitrile. The preparation method for the intermediate (II) includes the steps of carrying out acylation reaction between bromoacetic acid and III under the action of condensing agent so that IV is prepared, carrying out Grignard reaction between the IV and magnesium powder so that corresponding Grignard reagent V is prepared, and finally preparing the intermediate (II) via addition reaction between the Grignard reagent V and 2,4,5-trifluoro-benzeneacetonitrile in organic solvent. The preparation methods for the sitagliptin intermediates are mild in reaction conditions, simple and convenient in operation and available in raw materials and have excellent industrialized prospect.
Owner:SHANDONG BOYUAN PHARM CO LTD
Process method for synthesizing and purifying 4-chlorobutyryl chloride
InactiveCN104592001AHigh activityHigh selectivityChemical recyclingCarboxylic acid halides preparationPtru catalystSulfite salt
The invention discloses a process method for synthesizing and purifying 4-chlorobutyryl chloride. The process method comprises the following steps of reacting gamma-butyrolactone and thionyl chloride in the presence of a mixed catalyst to synthesize a 4-chlorobutyryl chloride crude product and purifying the 4-chlorobutyryl chloride crude product to obtain the 4-chlorobutyryl chloride finished product of which the purity is equal to or greater than 99%, the individual impurity is less than 0.30% and the yield is equal to or greater than 90%. In the process method, exhaust gas generated in the production of 4-chlorobutyryl chloride is comprehensively utilized to produce a 30% hydrochloric acid and sodium sulfite solution and the tail material is used for producing 4-chlorobutyric acid ester. The process method is simple, convenient to operate, high in yield and purity of a product and almost free of emission of three wastes and is environmentally friendly.
Owner:HUBEI BIOCHEM PHARMA TECH
Absorbent article comprising a fragrance or odor control composition
An absorbent article selected from a sanitary napkin, an incontinence pad and a pantyliner, comprises a topsheet layer, a backsheet layer, optionally one or more intermediate layers enclosed between the topsheet and the backsheet, a fastening adhesive applied on said backsheet garment facing surface and a liquid fragrance or odor control composition applied on or within a layer of said absorbent article. The liquid fragrance or odor control composition comprises from 5 to 50% wt of one or more esters selected from menthyl acetate, menthyl lactate, menthyl propionate, menthyl butyrrate, cis-3-hexenyl acetate, methyl-dihydrojasmonate, methyl jasmonate, hexyl iso-butyrate, linalyl acetate, benzyl acetate, phenyl ethyl acetate and less than 5% wt of other esters. The resulting articles present reduced degradation of the fastening adhesive layer due to the migration of components of the fragrance or odor control composition into the fastening adhesive layer.
Owner:THE PROCTER & GAMBLE COMPANY
Micro-reaction continuous flow synthesis method of levocarnitine
ActiveCN111592466ASimple structureHigh yieldOrganic compound preparationOrganic chemistry methodsCombinatorial chemistryButyric acid ester
The invention provides a micro-reaction continuous flow synthesis method of levocarnitine. An existing preparation method has the defects of complicated operation, long reaction time, low yield and the like. According to the method, (R)-4-halogenated-3-hydroxybutyrate and trimethylamine are continuously subjected to quaternization and hydrolysis reaction in a micro-channel reactor in the presenceof an alkali to prepare the levocarnitine. The reaction time of the method is only several minutes, the yield is high, the technological process is easy and convenient to operate, and industrial production is easy.
Owner:FUDAN UNIV
Stain resistant and anti-drop metal flash paint for photo frames
InactiveCN105936777AImprove stain resistanceImprove anti-shedding effectAntifouling/underwater paintsPaints with biocidesEpoxyFumed silica
The invention discloses a stain resistant and anti-drop metal flash paint for photo frames. The raw materials include: fluorocarbon resin, a carboxymethyl cellulose acetate butyrate aqueous dispersion, a waterborne polyacrylate emulsion, organic silicon resin, epoxy resin E-12, aluminum powder, hydrophobic fumed silica, composite filler, ethylene glycol butyl ether, hexamethoxymethyl melamine, dimethylethanolamine, isopropyl alcohol, sodium hexametaphosphate, butyl acetate, a silane coupling agent KH-560, a dispersant, a leveling agent BYK-331, a wetting agent, a defoaming agent, xylene and deionized water. The metal flash paint provided by the invention has excellent stain resistance and anti-drop performance.
Owner:蚌埠市禹会区贵宾装饰材料商行
S-beta-hydroxybutyrate compounds and compositions enriched with S-enantiomer
ActiveUS11185518B2Few or no effectsReduce loadPowder deliveryMetabolism disorderHydroxybutyric acidPharmaceutical medicine
Ketogenic compositions include a mixture of optically pure S-beta-hydroxybutyrate salts and acid(s) or non-racemic mixture of beta-hydroxybutyrate salts and acid(s) enriched with the S-enantiomer. The S-beta-hydroxybutyrate enantiomer modulates the effect of ketone bodies in the subject and controls the rate at which ketosis is achieved. Beta-hydroxybutyric acid is more rapidly absorbed and utilized by the body than salts or esters, enhances taste, and reduces the need to include citric acid or other edible acids. Beta-hydroxybutyrate salts are more slowly absorbed and utilized by the body and can provide one or more electrolytes. Compositions for controlling ketone body level in a subject may contain a dietetically or pharmaceutically acceptable carrier and optically pure S-beta-hydroxybutyrate or non-racemic mixture enriched with S-beta-hydroxybutyrate, wherein the compositions contains from about 50.5% to 100% by enantiomeric equivalents of S-beta-hydroxybutyrate and from about 49.5% to 0% by enantiomeric equivalents of R-beta-hydroxybutyrate.
Owner:AXCESS GLOBAL SCI LLC
Antibacterial coating layer and preparation technology thereof
InactiveCN108864880ABroad absorption spectrumHigh bactericidal activityAntifouling/underwater paintsPaints with biocidesKetoneDodecylbenzene
Owner:太仓斯迪克新材料科技有限公司
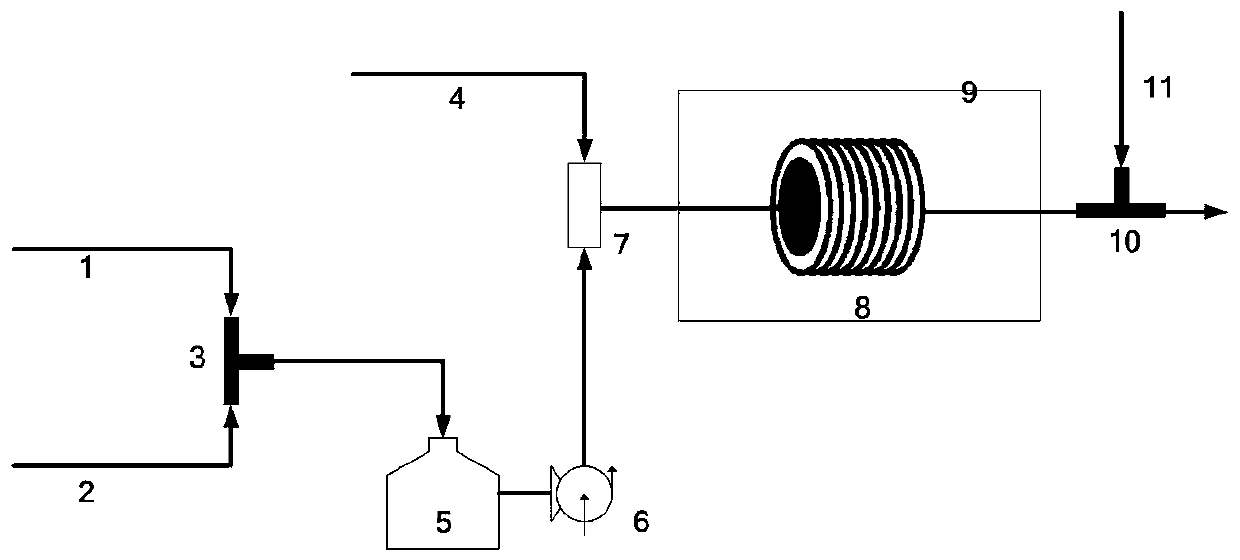
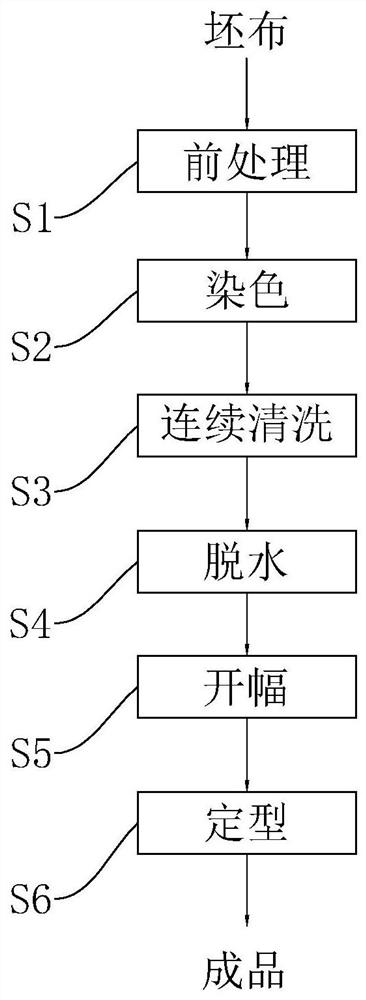
Dyeing process of polyester fabric
The application relates to the technical field of fabric dyeing and finishing, and discloses a dyeing process for polyester fabrics. A polyester grey fabric is subjected to the following operations: S1: pretreatment; S2: dyeing, namely, dyeing the grey fabric after the treatment in S1, wherein a dye used for dyeing comprises the following components in parts by weight: 20-40 parts of a disperse dye, 13-27 parts of a dispersant, 4-10 parts of 2,3-butanediol, 5-14 parts of cetyl ethyl hexanoate, 3-19 parts of carboxymethyl cellulose acetate butyrate, 20-40 parts of hydroxyethylidene diphosphonicacid, 20-40 parts of octadecylisocyanate, 3-9 parts of acetic acid and 230-310 parts of water; S3: continuous cleaning, namely, continuously cleaning the grey fabric after the treatment in S2; S4: dehydration; S5: scutching; and S6: setting; and the grey fabric after the setting in S6 is a dyed polyester fabric. The color fastness of polyester fabric dyeing can be improved.
Owner:诸暨市泓宇化纤漂染有限公司
Preparation method of chiral 4-hydroxy-4 phenyl butyric acid ester
InactiveCN102925500AEfficient asymmetric reduction reactionEasy to synthesizeMicroorganism based processesFermentationPhenylbutyrateBioconversion
The invention discloses a preparation method of chiral 4-hydroxy-4 phenyl butyric acid ester. The method comprises that candida magnoliae (Candida magnoliae CGMCC2.1919) or saccharomyces cerevisiae (saccharomyces cerevisiae CGMCC2.399) are marked on a peptone-yeast-glucose (PYG) medium flat plate which is sterilized at the temperature of 121 DEG C for 20min, the candida magnoliae and the saccharomyces cerevisiae are subjected to stationary culture at the temperature of 25-30 DEG C for 1 day, a single colony is picked out, the single colony serves as a seed and is subjected to inclined-plane stationary culture, and after the seed grows for two days, the seed is stored at the temperature of 4 DEG C for stand-by use; and conventional cell culture and conventional bioconversion are achieved. An obtained product is high in optical purity, low in cost, safe and environment-friendly.
Owner:ZUNYI MEDICAL UNIVERSITY
Complex of angiotensin receptor antagonist and neutral endopeptidase inhibitor
ActiveUS10537555B2Easy to controlLow hygroscopicityOrganic chemistry methodsCardiovascular disorderChronic heart diseasePropanoic acid
Provided a complex of formula is [3-((1S, 3R)-1-biphenyl-4-ylmethyl-3-ethoxycarbonyl-1-butylcarbamoyl) propionate-(S)-3′-methyl-2′-(pentanoyl {2″-(tetrazol-5-ylate) biphenyl-4′-ylmethyl}amino) butyrate]6.XCa2+.YNa+.ZH2O, wherein X=1-3, Y=12-16, Z=9-18, and 2X+Y=18, and represented by formula (I). Also disclosed are the method of preparing the complex and the method of treating chronic heart disease using a medicament comprising the complex.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
Synthesis process of 2, 2, 4-trimethyl-1, 3pentanediol dibutyrate and application of 2, 2, 4-trimethyl-1, 3pentanediol dibutyrate in interior wall latex paint
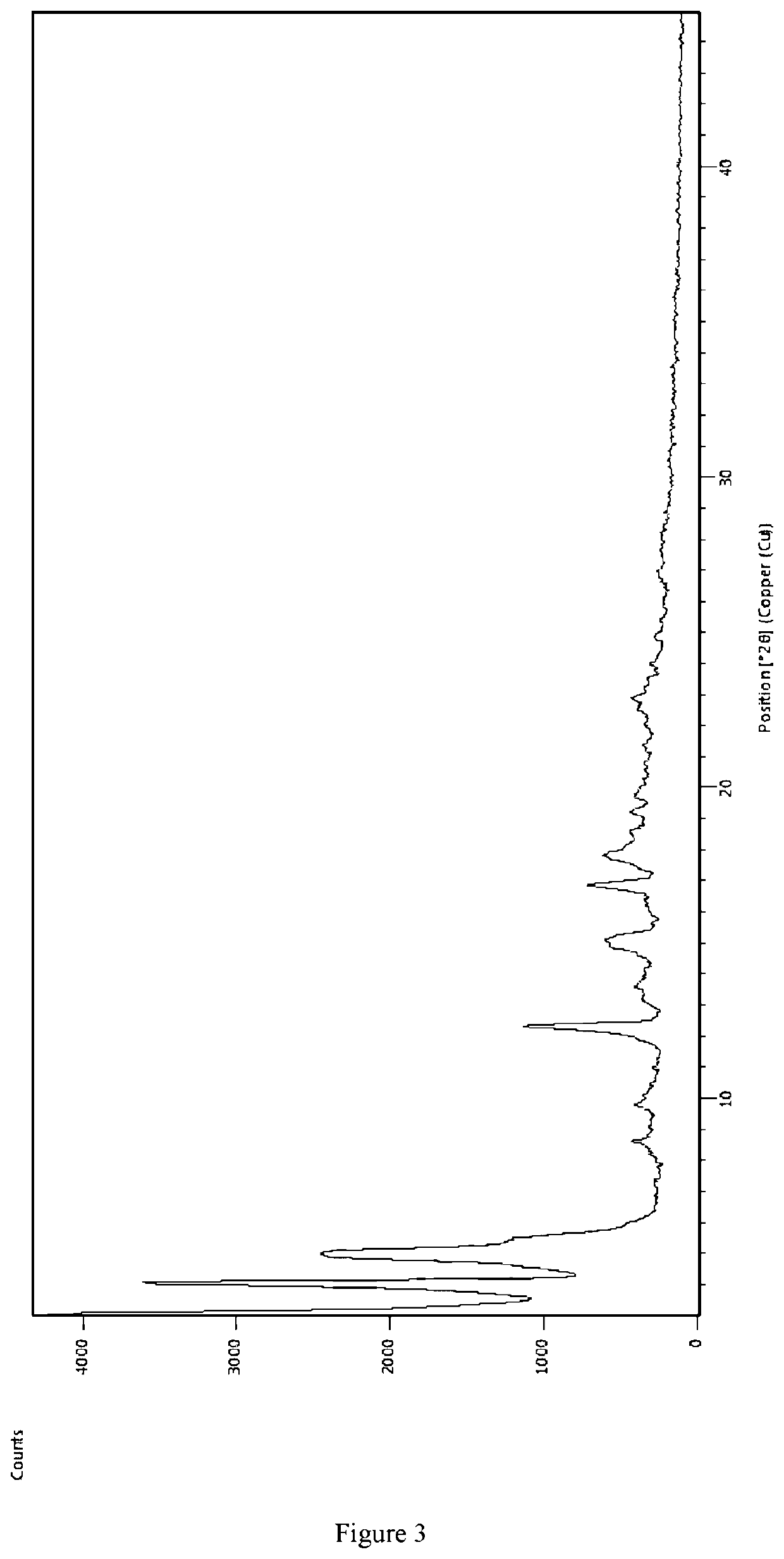
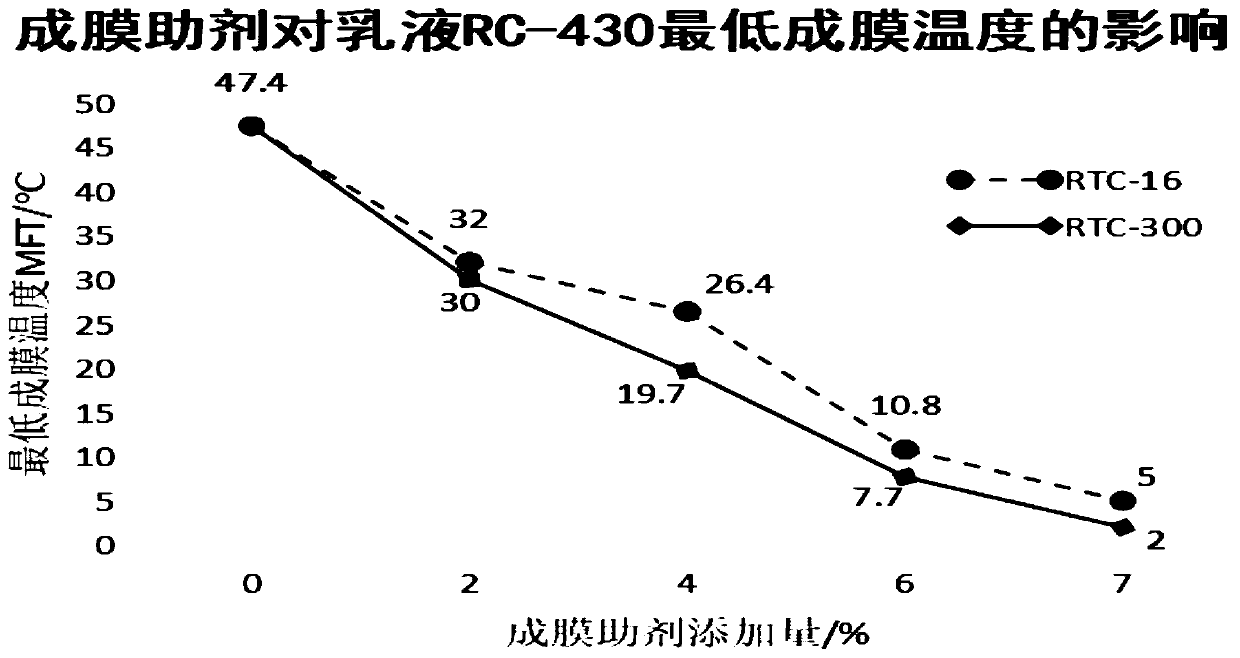
PendingCN111393288AHigh yieldLow film forming temperatureOrganic compound preparationCarboxylic acid esters preparationButyratePtru catalyst
The invention discloses a synthesis process of 2, 2, 4-trimethyl-1, 3pentanediol dibutyrate and application of 2, 2, 4-trimethyl-1, 3pentanediol dibutyrate in interior wall latex paint. According to the method, 2, 2, 4-trimethyl-1, 3pentanediol and butyric acid are used as the raw materials for reaction in the presence of a catalyst to prepare 2, 2, 4-trimethyl-1, 3pentanediol dibutyrate. According to the invention, 2, 2, 4-trimethyl-1, 3pentanediol dibutyrate is used as a film-forming assistant to prepare paint for the first time, high film forming efficiency is achieved, the RTC-300 adding amount in a same formula can be reduced by 10% compared with RTC-16, and the boiling point of the 2, 2, 4-trimethyl-1, 3pentanediol dibutyrate is higher, therefore the environmental protection requirement of TVOC is met.
Owner:RUNTAI CHEM TAIXING CO LTD
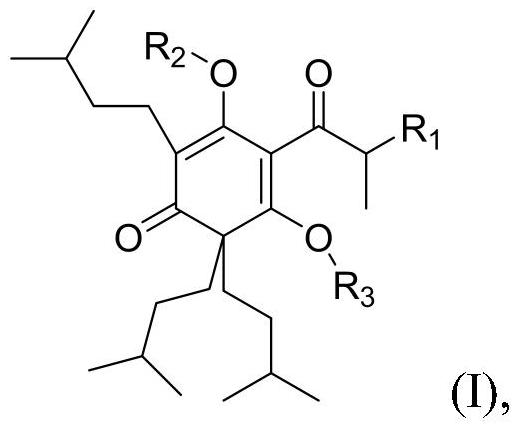
Precursor compound of hexahydro-beta-acid component compound, feed composition and application thereof
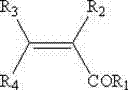
PendingCN112500289AOvercoming the problem of active ingredient degradationImprove stabilityPreparation from carboxylic acid halidesOrganic compound preparationBiotechnologyPropionate
The invention discloses a precursor compound of a hexahydro-beta-acid component compound, a feed composition and an application thereof. The invention relates to a precursor compound of a hexahydro-beta-acid component compound, or a solvate of the precursor compound and a feed acceptable salt, the structure of the precursor compound is shown as a formula (I), R1 is selected from substituted or non-substituted C1-C2 alkyl, and R2 or R3 is independently selected from H and substituted or unsubstituted straight-chain or branched-chain C2-C4 carbonyl. According to the invention, the fatty acid esterification precursor compound of the hexahydro-beta acid-component compound is found to have good stability under a high-temperature condition, and the problem of degradation of effective componentscaused by high-temperature granulation of the hexahydro-beta-acid component compound in a feed processing process is solved. Further, the invention also finds that the hexahydro-beta acid component compound propionate or butyrate and the feed acceptable salt or solvate thereof in the fatty acid esterification precursor compound of the hexahydro-beta acid component compound, not only can bear the high temperature course of feed processing, but also has the equivalent effect of the hexahydro-beta acid component compound when being applied to breeding.
Owner:GUANGZHOU INSIGHER BIOTECHNOLOGY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations](https://images-eureka.patsnap.com/patent_img/eb239ae2-f451-4c9e-8101-694067a7ab8d/US08877938-20141104-D00000.png)
![Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations](https://images-eureka.patsnap.com/patent_img/eb239ae2-f451-4c9e-8101-694067a7ab8d/US08877938-20141104-D00001.png)
![Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations](https://images-eureka.patsnap.com/patent_img/eb239ae2-f451-4c9e-8101-694067a7ab8d/US08877938-20141104-C00001.png)
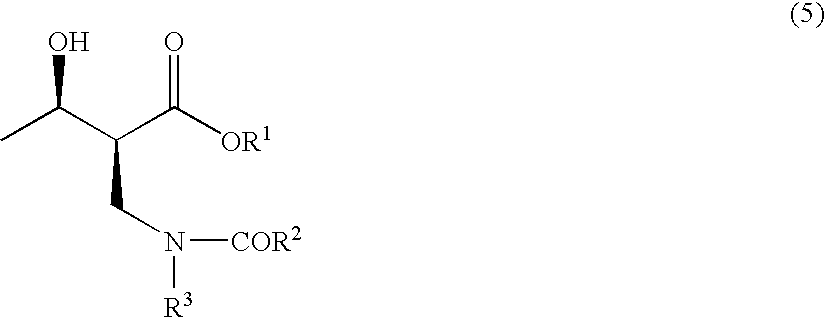
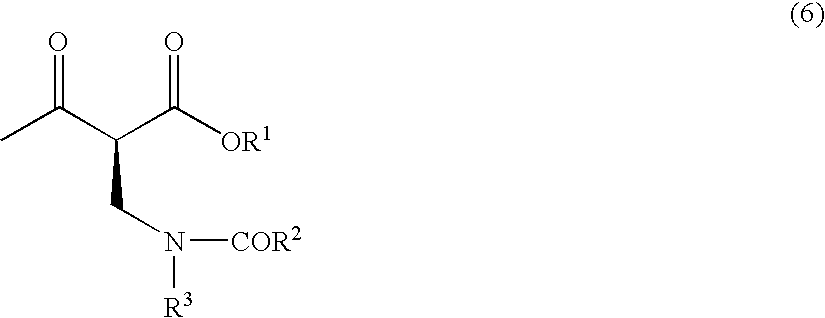
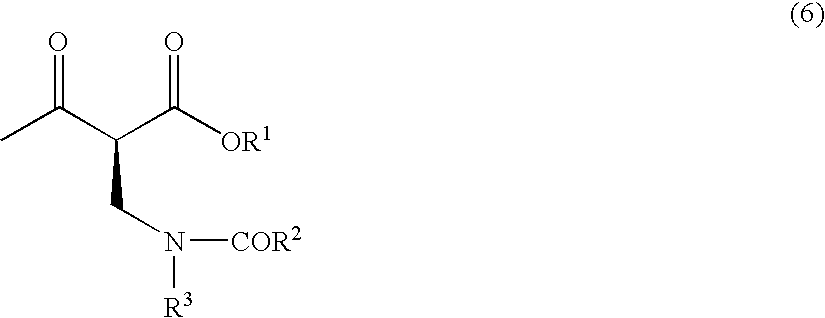
![SYNTHETIC METHODS FOR PREPARATION OF (S)-(2R,3R,11bR)-3-ISOBUTYL-9,10-DIMETHOXY-2,3,4,6,7,11b-HEXAHYDRO-1H-PYRIDO[2,1-a]ISOQUINOLIN-2-YL 2-AMINO-3-METHYLBUTANOATE DI(4-METHYLBENZENESULFONATE) SYNTHETIC METHODS FOR PREPARATION OF (S)-(2R,3R,11bR)-3-ISOBUTYL-9,10-DIMETHOXY-2,3,4,6,7,11b-HEXAHYDRO-1H-PYRIDO[2,1-a]ISOQUINOLIN-2-YL 2-AMINO-3-METHYLBUTANOATE DI(4-METHYLBENZENESULFONATE)](https://images-eureka.patsnap.com/patent_img/af5a93d0-aa6a-4674-8cd4-51408de0087f/US20170183346A1-20170629-C00001.png)
![SYNTHETIC METHODS FOR PREPARATION OF (S)-(2R,3R,11bR)-3-ISOBUTYL-9,10-DIMETHOXY-2,3,4,6,7,11b-HEXAHYDRO-1H-PYRIDO[2,1-a]ISOQUINOLIN-2-YL 2-AMINO-3-METHYLBUTANOATE DI(4-METHYLBENZENESULFONATE) SYNTHETIC METHODS FOR PREPARATION OF (S)-(2R,3R,11bR)-3-ISOBUTYL-9,10-DIMETHOXY-2,3,4,6,7,11b-HEXAHYDRO-1H-PYRIDO[2,1-a]ISOQUINOLIN-2-YL 2-AMINO-3-METHYLBUTANOATE DI(4-METHYLBENZENESULFONATE)](https://images-eureka.patsnap.com/patent_img/af5a93d0-aa6a-4674-8cd4-51408de0087f/US20170183346A1-20170629-C00002.png)
![SYNTHETIC METHODS FOR PREPARATION OF (S)-(2R,3R,11bR)-3-ISOBUTYL-9,10-DIMETHOXY-2,3,4,6,7,11b-HEXAHYDRO-1H-PYRIDO[2,1-a]ISOQUINOLIN-2-YL 2-AMINO-3-METHYLBUTANOATE DI(4-METHYLBENZENESULFONATE) SYNTHETIC METHODS FOR PREPARATION OF (S)-(2R,3R,11bR)-3-ISOBUTYL-9,10-DIMETHOXY-2,3,4,6,7,11b-HEXAHYDRO-1H-PYRIDO[2,1-a]ISOQUINOLIN-2-YL 2-AMINO-3-METHYLBUTANOATE DI(4-METHYLBENZENESULFONATE)](https://images-eureka.patsnap.com/patent_img/af5a93d0-aa6a-4674-8cd4-51408de0087f/US20170183346A1-20170629-C00003.png)