Micro-reaction continuous flow synthesis method of levocarnitine
A technology of micro-reaction and micro-channel reactor, applied in the direction of organic chemical method, cyanide reaction preparation, chemical instrument and method, etc., can solve the problems of many side reactions, low yield, long reaction time, etc., and achieve simple operation, Increased product yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
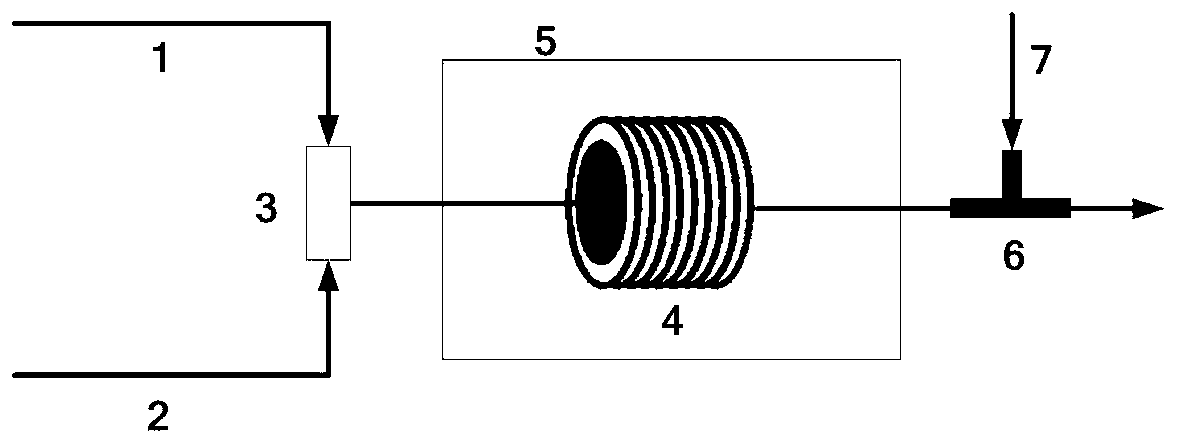
[0043] The microchannel reactor adopts a 316L stainless steel microtubular reactor with an outer diameter of 1.6 mm, an inner diameter of 1.0 mm, and a total volume of 7.85 ml. For the reaction process, see figure 1 .
[0044]Firstly prepare an aqueous sodium hydroxide solution with a concentration of 4.77% by mass and an aqueous solution of hydrochloric acid with a concentration of 10% by mass, then pass trimethylamine gas into the aqueous solution of sodium hydroxide to prepare a solution with a concentration of 12% by mass. Trimethylamine aqueous solution. The trimethylamine aqueous solution and the substrate (R)-(+)-4-chloro-3-hydroxybutyrate ethyl ester are simultaneously pumped into the micro-mixer (3), and the volume flows of the two materials are adjusted respectively so that the substrate and the trimethylamine The mol ratio is 0.25, and two strands of materials are mixed in the micro-mixer (3), then enter the micro-channel reactor (4), the temperature in the micro-...
Embodiment 2
[0046] The microchannel reactor adopts a polytetrafluoroethylene (PTFE) microtubular reactor with an outer diameter of 1.6 mm, an inner diameter of 0.9 mm, and a total volume of 9.54 ml. For the reaction process, see figure 1 .
[0047] First prepare the aqueous sodium hydroxide solution with a mass percentage concentration of 6.34% and the hydrochloric acid aqueous solution with a mass percentage concentration of 8%, then pass trimethylamine gas into the above-mentioned aqueous sodium hydroxide solution to prepare a 15% aqueous solution of Trimethylamine aqueous solution. The trimethylamine aqueous solution and the substrate (R)-(+)-4-chloro-3-hydroxybutyrate ethyl ester are simultaneously pumped into the micro-mixer (3), and the volume flows of the two materials are adjusted respectively so that the substrate and the trimethylamine The mol ratio is 0.3, and the two stocks of materials are mixed in the micro-mixer (3), then enter the micro-channel reactor (4), the temperatu...
Embodiment 3
[0049] The microchannel reactor adopts a 304 stainless steel microtubular reactor with an outer diameter of 3.2 mm, an inner diameter of 2.2 mm, and a total volume of 38 ml. For the reaction process, see figure 1 .
[0050] First prepare the aqueous sodium hydroxide solution with a mass percentage concentration of 3.6% and the hydrochloric acid aqueous solution with a mass percentage concentration of 7%, then pass trimethylamine gas into the above-mentioned aqueous sodium hydroxide solution, and prepare a 8% hydrochloric acid solution with a mass percentage concentration of Trimethylamine aqueous solution. The trimethylamine aqueous solution and the substrate (R)-(+)-4-chloro-3-hydroxybutyrate ethyl ester are simultaneously pumped into the mixer (3), and the volume flows of the two stocks of materials are adjusted respectively so that the substrate and the trimethylamine The molar ratio is 0.32. The two materials are mixed in the micro-mixer (3), and then enter the micro-ch...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Outer diameter | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com