Application of arctigenin valine ester hydrochloride to preparation of anti-tumor drugs
The technology of methyl butyrate and application is applied in the application field of arctigenin valine ester hydrochloride in the preparation of antitumor drugs, and can solve the problems of low oral bioavailability, restricted application and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
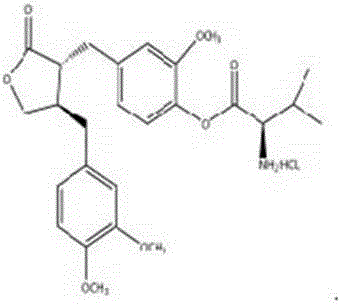
[0012] Example 1 4-(4-(3,4-dimethoxybenzyl)-2-carbonyl)tetrahydrofuranmethyl-2-methoxyphenol 2-amino-3-methylbutyrate hydrochloride synthesis.
[0013] Weigh 0.20g (0.54mmol) arctigenin, 0.23g (1.08mmol) Boc-L-valine, 0.21g (1.08mmol) 1-ethyl-(3-dimethylaminopropyl) carbon diacetate Put imine hydrochloride (EDCI), 0.03g (0.27mmol) 4-dimethylaminopyridine (DMAP) in a 100ml spinner bottle, add 10ml of acetonitrile solution, stir and dissolve in an ice-water bath, and react at room temperature for 1-2 hours. The reaction was detected by TLC until the reaction was complete, and the solvent was evaporated under reduced pressure to obtain a light yellow viscous substance. The viscous material was separated by column chromatography with YMC reverse-phase packing, and eluted with acetonitrile / water (55:45) mixed solvent to collect the desired components, recover the organic solvent, and freeze-dry to obtain a white powdery compound. Dissolve the white powdery compound obtained above...
Embodiment 2
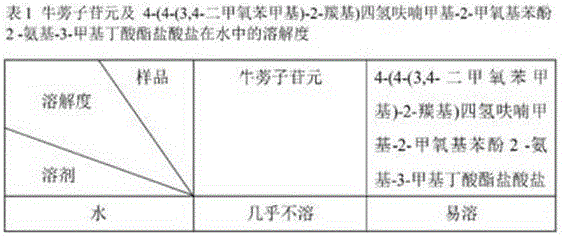
[0014] Example 2 Arctigenin and 4-(4-(3,4-dimethoxybenzyl)-2-carbonyl)tetrahydrofuranmethyl-2-methoxyphenol 2-amino-3-methylbutanoic acid The solubility of ester hydrochloride in water is shown in Table 1.
[0015]
[0016] As can be seen from Table 1, after arctigenin is structurally modified, its modified product 4-(4-(3,4-dimethoxybenzyl)-2-carbonyl)tetrahydrofurylmethyl-2-methoxy The water solubility of phenol 2-amino-3-methylbutyrate hydrochloride is greatly increased, which improves the shortcoming of arctigenin's low solubility and helps to improve the bioavailability.
Embodiment 3
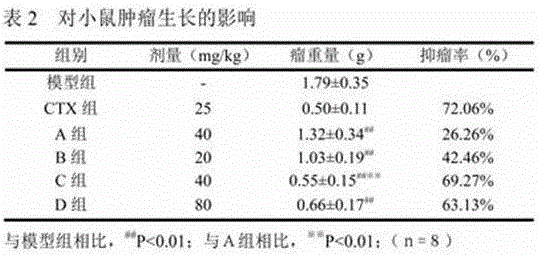
[0017] Example 3 4-(4-(3,4-dimethoxybenzyl)-2-carbonyl)tetrahydrofuranmethyl-2-methoxyphenol 2-amino-3-methylbutyrate hydrochloride Tumor suppression and immune function in H22 xenografted mice.
[0018] 1. Materials and methods
[0019] 1.1 Materials
[0020] 1.1.1 Experimental animals and cell lines
[0021] SPF grade ICR mice (male), weighing 19-21 g, animal certificate number: SCXK (Ji)-2011-0004, were purchased from Changchun Yisi Experimental Animal Technology Co., Ltd. Mouse quality inspection unit: Jilin Provincial Laboratory Animal Quality Inspection Center.
[0022] 1.1.2 Drugs and reagents
[0023] Arctigenin extraction and separation preparation
[0024] Synthesis and preparation of arctigenin valine ester hydrochloride
[0025] Cyclophosphamide (CTX) Shanghai Hualian Pharmaceutical Co., Ltd.
[0026] Burea Nitrogen (BUN) Test Kit Nanjing Jiancheng Bioengineering Research Institute
[0027] Creatinine (Cr) Test Kit Nanjing Jiancheng Bioengineering Institute...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com