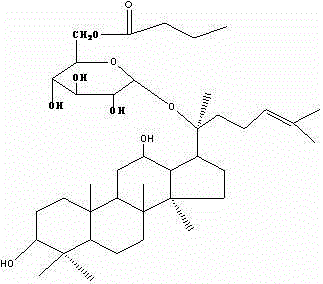
Preparation of ginsenoside M1 n-butyrate and application of antidiabetic medicines of ginsenoside M1 n-butyrate
A technology of n-butyrate and ginsenoside, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, metabolic diseases, etc., can solve problems such as anti-diabetic literature reports, and achieve less impurities, high yield, and substrate fully responsive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] a) Dissolve 1.0g of M1 in 250mL THF, fully dissolve, add 10mL of 10mg / mL DMAP solution in THF to the reaction solution, incubate for 3-5min, then slowly add 500 mg of n-Butyric anhydride to the system, react 0.1-1h; after the reaction is completed, the reaction solution is concentrated, and the dry matter is dissolved with ethyl acetate, then washed with water and saturated brine in sequence, and the ethyl acetate is recovered under reduced pressure to obtain a dry matter.
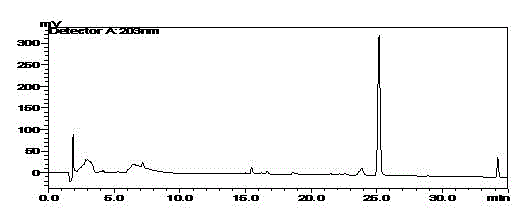
[0037] b) The dry matter was dissolved in a small amount of methanol, filtered, and subjected to silica gel column chromatography, eluting with chloroform-methanol (30:1-5:1), followed by TLC; the target products were combined and recovered under reduced pressure to obtain 0.723 g of dry matter. That is, ginsenoside M1 monoester n-BM1, in which fatty acyl is C 4 The fatty acyl group (the fatty acyl group is connected to the C’6 position of the sugar chain of the C-20 glucose group), see HPLC figur...
Embodiment 2
[0039] a) Weigh 500mg of ginsenoside M1, dissolve it in 150mL THF, fully dissolve, add 5mL of 5mg / mL DMAP solution in THF to the reaction solution, incubate for 3-5min, then slowly add 250 mg of n-Butyric anhydride into the system , react for 0.1-1h; after the reaction, concentrate the reaction solution, dissolve the dry matter with ethyl acetate, then sequentially water, saturated brine, and recover the ethyl acetate under reduced pressure to obtain dry matter.
[0040] b) The dry matter was dissolved in methanol, filtered, and subjected to silica gel column chromatography, eluting with chloroform-methanol (30:1-5:1), followed by TLC; the target products were combined and recovered under reduced pressure to obtain 0.35 g of dry matter. That is, ginsenoside M1 monoester n-BM1, in which fatty acyl is C 4 The fatty acyl group (the fatty acyl group is connected to the C’6 position of the sugar chain of the C-20 glucose group), see HPLC figure 1 .
Embodiment 3
[0042]a) Weigh 750mg of ginsenoside M1, dissolve it in 200mL THF, fully dissolve, add 8mL of 7.5mg / mL DMAP solution in THF to the reaction solution, incubate for 3-5min, then slowly add n-Butyric anhydride 400 into the system mg, reacted for 0.1-1h; after the reaction was completed, the reaction solution was concentrated, and the dry matter was dissolved with ethyl acetate, then washed with water and saturated brine in sequence, and the ethyl acetate was recovered under reduced pressure to obtain a dry matter.
[0043] b) The dry matter was dissolved in methanol, filtered, and subjected to silica gel column chromatography, eluting with chloroform-methanol (30:1-5:1), followed by thin-layer chromatography; the target products were combined and recovered under reduced pressure to obtain 0.5 g of dry matter. That is, ginsenoside M1 monoester n-BM1, in which fatty acyl is C 4 The fatty acyl group (the fatty acyl group is connected to the C’6 position of the sugar chain of the C-20...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com