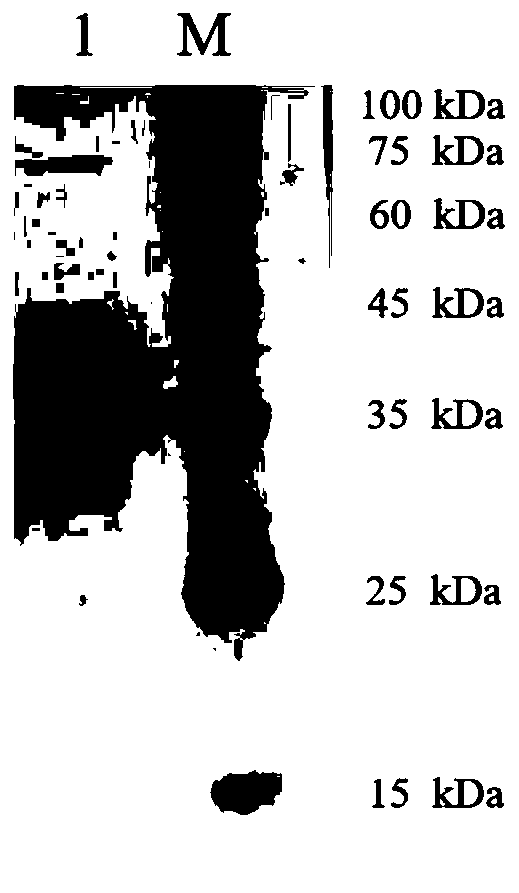
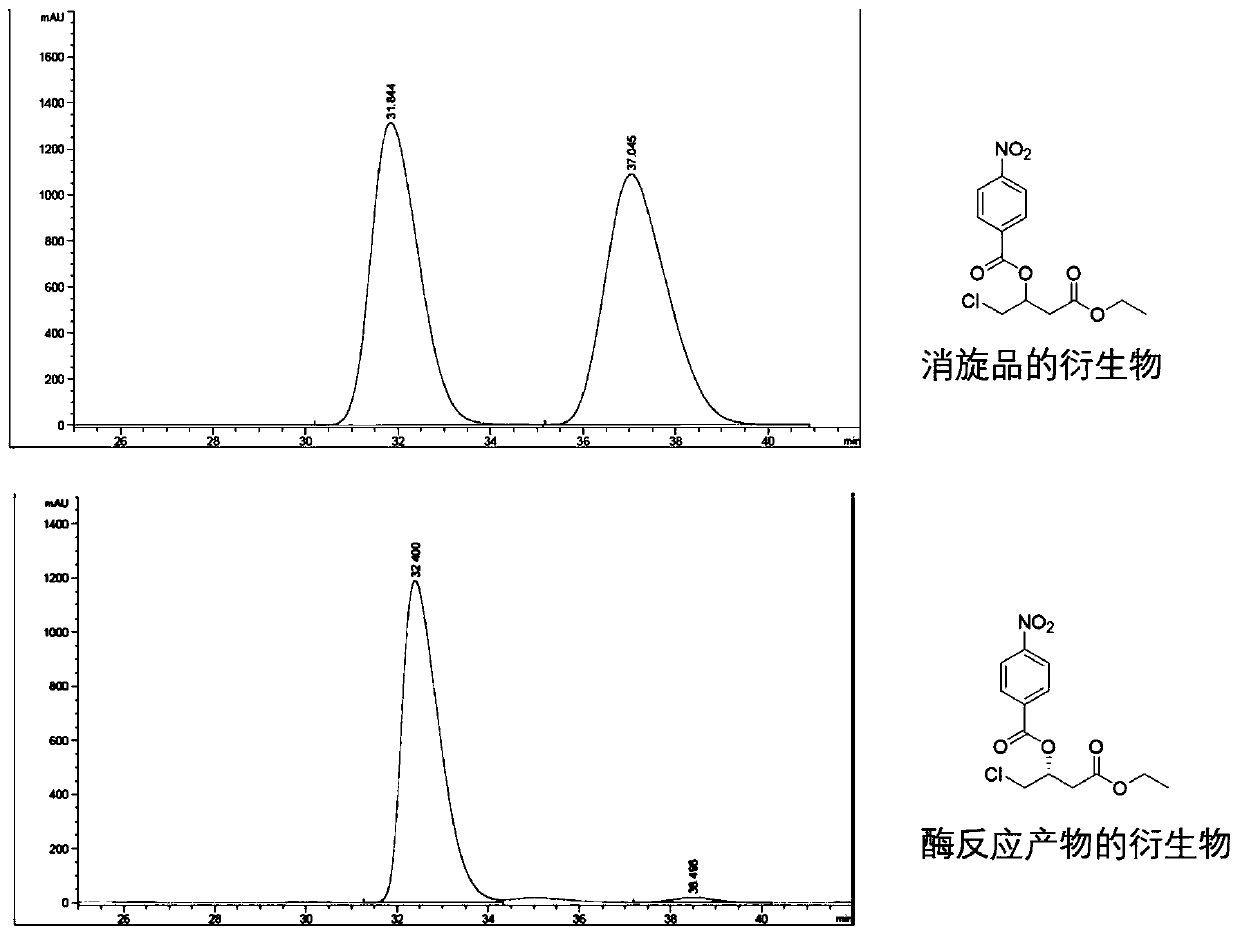
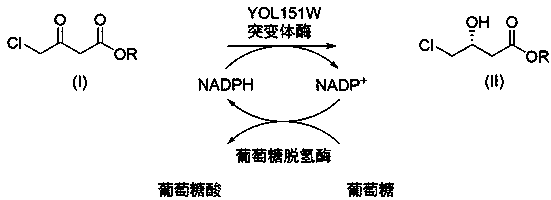
Carbonyl reductase mutant and application thereof in preparation of (R)-4-chloro-3-hydroxy-butyrate
A reductase and mutant technology, applied to carbonyl reductase mutants and their application fields in the preparation of (R)-4-chloro-3-hydroxy-butyrate, can solve the problems of low substrate concentration and the like, Achieve high substrate concentration, good industrial application prospects, and high stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]Example 1, site-directed mutagenesis and recombinant expression vector pET28b-YOL151W F85M build
[0031] Site-directed mutagenesis was performed using TransStart FastPfu Fly DNA polymerase. First design mutation primers containing mutation point F85M:
[0032] Upstream primer: GGCCTCTCCAATGTGCTTTGATATCACTGACAGT
[0033] Downstream primer: TATCAAAGCACATTGGAGAGGCCGTATGTAGAAC;
[0034] PCR reaction system (50μL): wild-type pET28b-YOL151W template 50ng, 10μL 5×TransStart ® FastPfuFly Buffer, 8μL dNTPs (2.5mM each), 1μL (10μM) each of a pair of mutation primers, 10μL 5×PCR Stimulant, 2.5 units of TransStart FastPfu Fly DNA polymerase, add sterile distilled water to 50μL.
[0035] PCR amplification procedure: (1) denaturation at 98°C for 3 min; (2) denaturation at 98°C for 20s, (3) annealing at 65°C for 30s, (4) extension at 72°C for 8 min, steps (2)-(4) were carried out for a total of 20 cycles , the final extension at 72 °C for 10 min, and the product was stored at 4 °...
Embodiment 2
[0037] Example 2, genetically engineered bacteria E.coli BL21(DE3) / pET28b-YOL151W F85M Construction and inducible expression of / pACYC-GDH
[0038] Using the plasmid pET28b-YOL151W constructed in Example 1 F85M and laboratory-preserved pACYC-GDH to co-transform the expression host E. coli BL21(DE3), positive clones were obtained by screening, and the engineering bacteria were named as E. coli BL21(DE3) / pET28b-YOL151W F85M / pACYC-GDH. The engineered bacteria were inoculated into 5 mL of LB liquid medium containing kanamycin (25 μg / mL) and chloramphenicol (12.5 μg / mL) for activation for 8 h (37 °C, 200 rpm). Take the above activated culture and transfer it to 500 mL of LB liquid medium containing kanamycin (25 μg / mL) and chloramphenicol (12.5 μg / mL) at 1 / 100 of the inoculum size (37 °C, 200 rpm). ), when the absorbance density OD of the culture medium 600 When it reached 0.6, 0.1 mM IPTG was added for induction, the induction temperature was 18°C, and the induction ti...
Embodiment 3
[0039] Embodiment 3, engineering bacteria E. coli BL21(DE3) / pET28b-YOL151W F85M / pACYC-GDH whole-cell catalyzed asymmetric synthesis ( R )-4-chloro-3-hydroxy-butyric acid ethyl ester (100g grade)
[0040] Ethyl chloroacetoacetate (346.8 g) was added to the reaction kettle and stirred. Toluene (534 mL) was added and the temperature was maintained at 30 °C. Glucose (570.9 g) was added and stirred for 5 minutes. On the other hand, 260 g of the whole-cell catalyst was added to 1.2 L of 100 mM phosphate buffer (pH 6.7), and the mixture was stirred uniformly, and this bacterial slurry suspension was added to the above-mentioned toluene reaction mixture. The pH was monitored from time to time after the reaction started, using 2M K 2 CO 3 The solution was maintained at pH 6.7. When the GC-MS monitoring reaction was completed, 300 mL of ethyl acetate was added, and after stirring for 5 minutes, the reaction solution was centrifuged at 9500 rpm for 20 minutes. The organic phase...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com