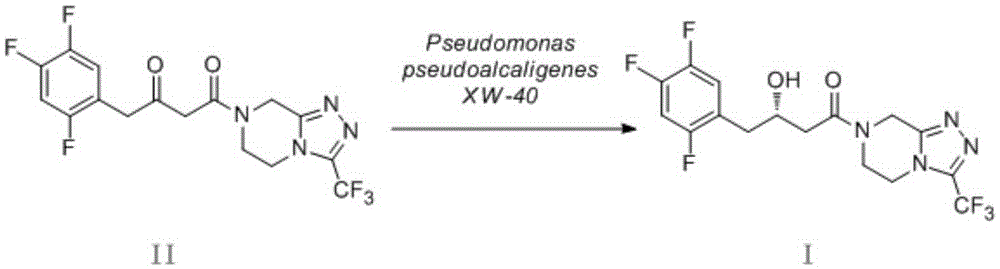
Pseudomonas pseudoalcaligenes and application of pseudomonas pseudoalcaligenes to preparation of sitagliptin intermediate
The technology of pseudomonas and sitagliptin is applied to pseudomonas alkaloids strain and its application field in the preparation of sitagliptin intermediates, which can solve the problems of easy inactivation of enzymes, excessively long synthetic route, It is not suitable for problems such as large-scale industrial production, and achieves the effect of weak substrate inhibition and simplified separation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
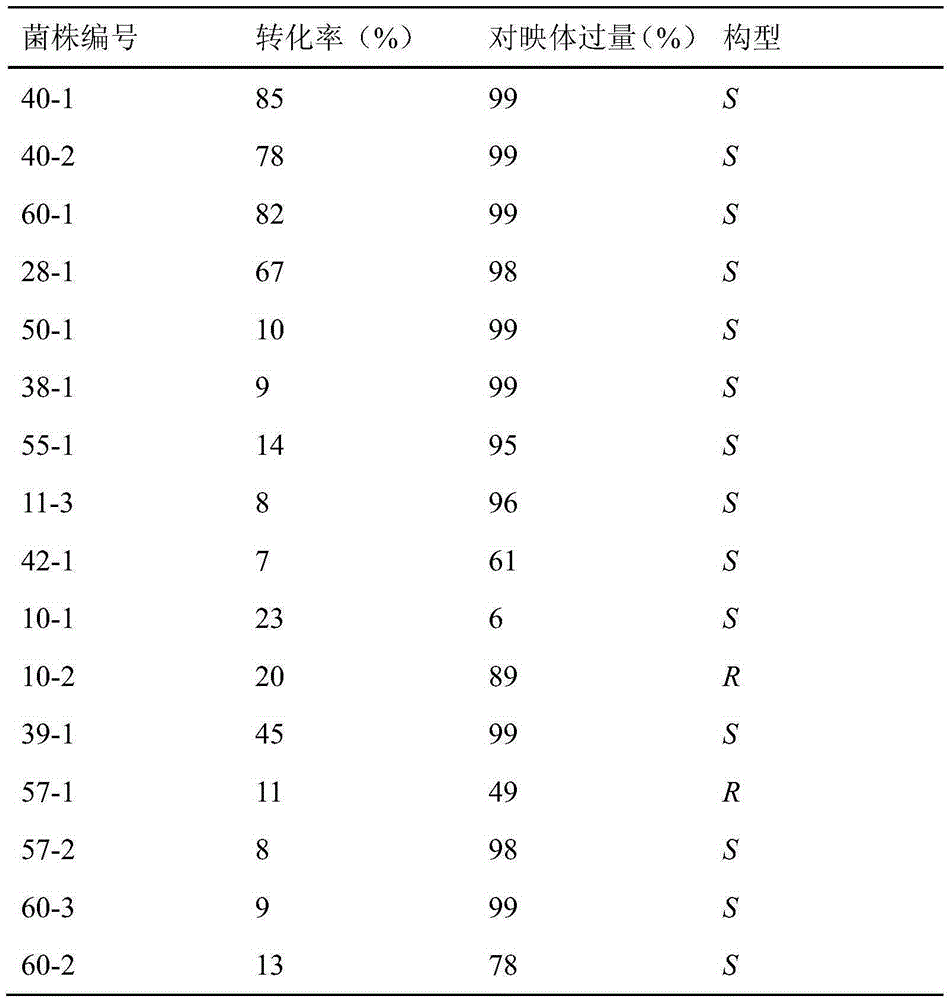
[0028] The primary screening of embodiment 1 soil bacterial strain
[0029] Weigh about 1g of soil sample into 10ml of sterile water, oscillate to mix and centrifuge, absorb 1ml of the supernatant and add it to 100ml of minimal salt medium with acetophenone as the only carbon source, and incubate at 30°C for 5-7 days. Then spread the cultured bacterium suspension to the minimum salt agar medium with acetophenone as the only carbon source, separate and purify the bacterium colonies grown on the plate and inoculate them on the agar slant (medium composition: peptone 5g / L, Yeast extract 1.5g / L, glucose 10g / L, beef extract 1.5g / L, NaCl 5g / L, pH 7.0), cultured at 30°C for 48h, as a suspected strain for further screening, numbered and preserved.
Embodiment 2
[0030] Embodiment 2 produces the screening of carbonyl reductase bacterial strain
[0031] The bacterial classification obtained by the isolation and purification of Example 1 is inoculated into peptone 5g / L, yeast extract 1.5g / L, glucose 10g / L, beef extract 1.5g / L, NaCl5g / L, pH7.0 medium, Cultivate at 30°C for 48h, centrifuge at 7000rpm / min at 4°C for 10min, collect the cells, wash twice with cold saline, and obtain cell-wet cells.
[0032]The following bacterial strain screening system is used for screening: 1g / L substrate (II), glucose with a mass fraction of 5%, 10% (v / v) absolute ethanol, 80g / L thalline (dry weight), 0.1M diphosphate Sodium hydrogen-disodium hydrogen phosphate buffer solution, pH 7.0, stirred and reacted at 30°C for 12h, centrifuged, the supernatant was extracted 3 times with ethyl acetate, the organic layers were combined, dried overnight with anhydrous magnesium sulfate, reversed phase C 18 Conversion and enantiomeric excess (ee) were determined by liq...
Embodiment 3
[0040] Embodiment 3 product (I) preparation
[0041] Preparation of (S)-3-hydroxy-1-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyridine by PseudomonaspseudoalcaligenesXW-40 bacteria Azin-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-1-one (I). Get 5ml of seed solution and transfer it to 100ml of fresh culture medium (shake flask with 250ml capacity) (medium components and culture conditions see Example 2), at 30°C, 190 rpm shaking culture for 48 hours, 4°C Centrifuge at 7000 rpm for 10 minutes, collect the precipitated bacteria, and wash twice with cold saline to obtain wet bacteria as a biocatalyst. The obtained wet thallus is made into the thalline concentration with the phosphate buffer solution of pH7.0 and is 50g (dry weight) / L bacterium suspension. 5ml reaction system, the concentration of substrate (II) is 1g / L, add 5% glucose by mass fraction as coenzyme regeneration substrate, 10% absolute ethanol by volume fraction as organic co-solvent, 30°C, 190 rpm, conver...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com