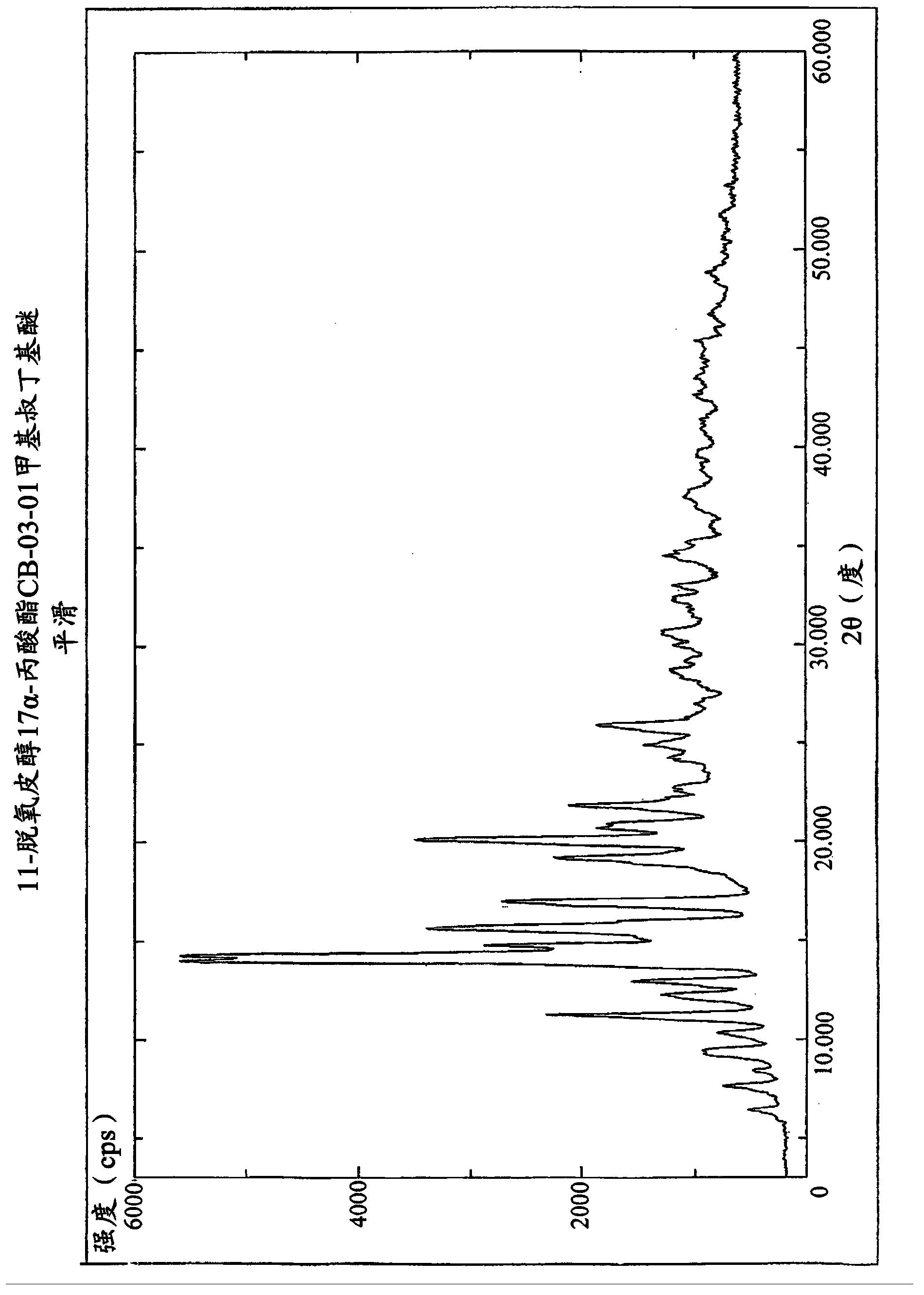
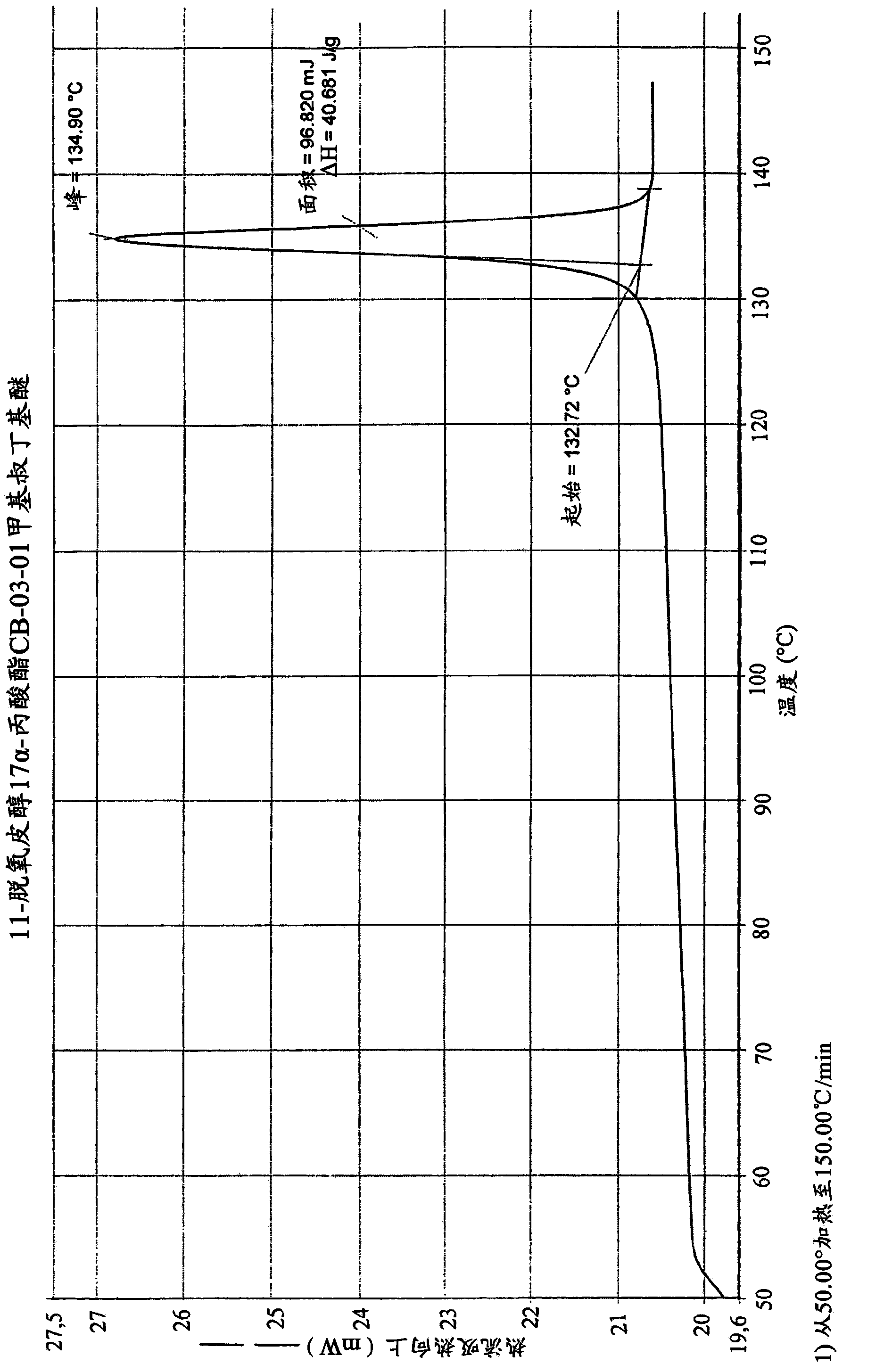
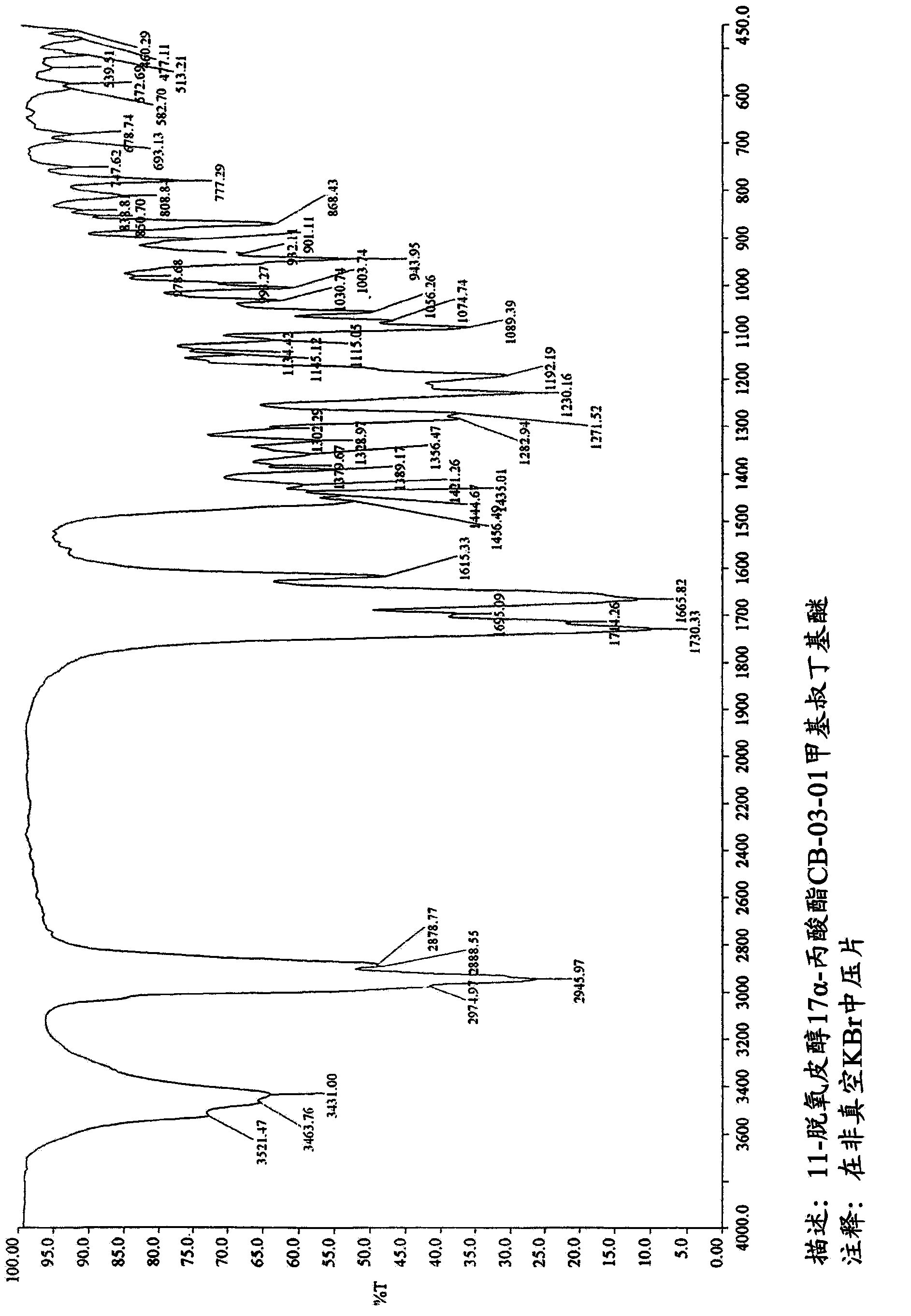
Enzymatic process for obtaining 17 alpha-monoesters of cortexolone and/or its 9,11-dehydroderivatives
A technology for deoxycortisol and medicine, which is applied in the field of enzymatic method for obtaining 17α-monoester of 11-deoxycortisol and/or 9,11-dehydroderivatives thereof, and can solve the problem that the monoester is unstable and difficult to obtain. Operation and separation issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Alcoholysis of 11-deoxycortisol 17α,21-dipropionate using CCL
[0094]Butanol (0.4 g, 5.45 mmol) and CCL (17.4 g, 3.86 U / mg, FLUKA) were added to 11-deoxycortisol-17α, 21-dipropionate (0.5 g, 1.09 mmol) in toluene ( 50ml) solution. The mixture was kept stirring at 30° C., then reacted in TLC (toluene / ethyl acetate 6 / 4) until the starting material dissolved (24 h). The enzyme was removed by filtration using a Celite bed. 11-Deoxycortisol 17α-propionate (0.437, 99%) was recovered after evaporation at low pressure. The product was obtained with a purity >99% in HPLC by crystallization from diisopropyl ether.
[0095] 1 H-NMR (500MHz, CDCl 3 ) correlation signal δ (ppm) 5.78 (br s, 1H, H-4), 4.32 (dd, 1H, H-21), 4.25 (dd, 1H, H-21), 1.22 (s, 3H, CH 3 -19),1.17(t,3H,CH 3 ),0.72(s,3H,CH 3 -18).P.f.114℃
Embodiment 2
[0097] 11-Deoxycortisol 17α-butyrate was prepared according to the method described in Example 1
[0098] 1 H-NMR correlation signal δ(ppm)5.78(br s,1H,H-4),4.32(dd,1H,H-21),4.26(dd,1H,H-21),1.23(s,3H,CH 3 -19),0.97(t,3H,CH 3 ),0.73(s,3H,CH 3 -18).P.F.134-136℃
Embodiment 3
[0100] 11-Deoxycortisol-17α-valerate was prepared according to the method described in the examples
[0101] 1 H-NMR correlation signal δ(ppm)5.77(br s,1H,H-4),4.32(dd,1H,H-21),4.26(dd,1H,H-21),1.22(s,3H,CH 3 -19),0.95(t,3H,CH 3 ),0.72(s,3H,CH 3 -18). P.f. 114°C (diisopropyl ether).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com