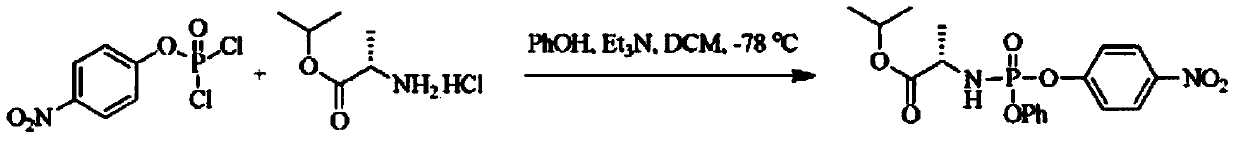Preparation method of 3,5-dibenzoyl-2-deoxy-2-fluoro-2-methyl-D-ribo-gamma-lactone
A technology of isopropyl dibenzoyl and phosphorylalanine, which is applied in the field of medicine and chemical industry, can solve the problems of production safety hazards and Chen Bengao, and achieve the goals of avoiding explosiveness, increasing safety and reducing production costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
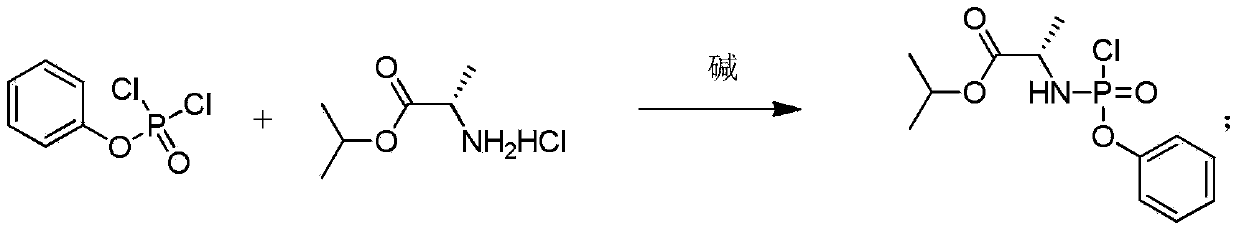
[0040] Example 1 Preparation of (S)-2-[(4-trifluoromethyl-phenoxy)-phenoxy-phosphorylamino]propionic acid isopropyl ester (±) under nitrogen / argon protection, in a circle Add 7.95g (47.4mmol) L-alanine isopropyl hydrochloride, 10.0g (47.4mmol) phenyl dichlorophosphate, and 100mL technical grade dichloromethane into the bottom reaction bottle, drop to -10°C, and Stir well at this temperature, then slowly add 13.2mL of triethylamine (95mmol) in 30mL of dichloromethane solution dropwise, keep the temperature not higher than -5°C, after the dropwise addition, slowly return to 5°C, and Stir at ℃ for 12-24 hours; then cool down to 0℃, add 7.29g of 4-trifluoromethylphenol (45.0mmol), then slowly add 6.28mL of triethylamine in 30mL of dichloromethane solution dropwise, keeping the temperature not higher than 5°C, after the dropwise addition, slowly return to room temperature, and stir at room temperature for 12-24 hours; add cold water, stir and separate layers, wash the organic layer...
Embodiment 2
[0041] Example 2 Preparation of (S)-2-[(S)-(4-trifluoromethyl-phenoxy)-phenoxy-phosphorylamino]propionic acid isopropyl ester Under nitrogen / argon protection, the Add 10.2g of crude product (S)-2-[(4-trifluoromethyl-phenoxy)-phenoxy-phosphorylamino]propanoic acid isopropyl ester (±) into 15mL of methyl tert-butyl ether, Then 45 mL of petroleum ether was added, cooled to -20°C, and stirred at this temperature for 12-24 hours. While cold filtered, the solid was washed with petroleum ether at -20°C to obtain (S)-2-[(S)-(4-trifluoromethyl-phenoxy)-phenoxy-phosphorylamino]propionic acid iso Propyl ester, further recrystallized with petroleum ether to obtain high-purity (S)-2-[(S)-(4-trifluoromethyl-phenoxy)-phenoxy-phosphorylamino]propionic acid isopropyl ester After drying, 2.58 g of the product was obtained, with a yield of 25.3%. 1H-NMR (CDCl 3 -d 6 ,400MHz)δ(ppm):1.226(d,3H,J=2.4Hz,-C H 3 ),1.242(d,3H,J=2.4Hz,-CH 3 ),1.393(d,3H,J=6.8Hz,-CH 3 ),3.873-3.926(m,1H,),4.063-4...
Embodiment 33
[0042] Example 33, Preparation of 5-dibenzoyl-2-deoxy-2-fluoro-2methyl-D-ribose-γ-lactone (Sofosbuvir) Under nitrogen protection, 100mg (2'R )-2'-deoxy-2'-fluoro-2'-methyluridine (0.384mmol) and 1.0mL of anhydrous tetrahydrofuran were added to the reaction flask, lowered to 0°C, and then slowly added dropwise 1.3M tert-butylmagnesium chloride solution (0.62 mL, 0.806 mmol, 2.1 equiv). After stirring for 30 min, 265 mg (S)-isopropyl 2-[(S)-(4-trifluoromethyl-phenoxy)-phenoxy-phosphorylamino]propionate (0.614 mmol) was added. The mixture was stirred at room temperature for 48 h, then washed with saturated NH 4 Quenched with aqueous Cl (10 mL). The mixture was partitioned between ethyl acetate 20 mL and water. The combined organic extracts were dried over anhydrous magnesium sulfate and concentrated. The residue was subjected to silica gel chromatography (200-300 mesh GF254 silica gel) using a 10-15% ethyl acetate / petroleum ether gradient method to obtain 75.6 mg of a white s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com