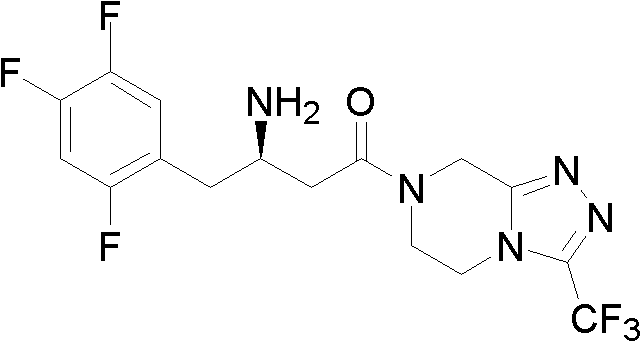
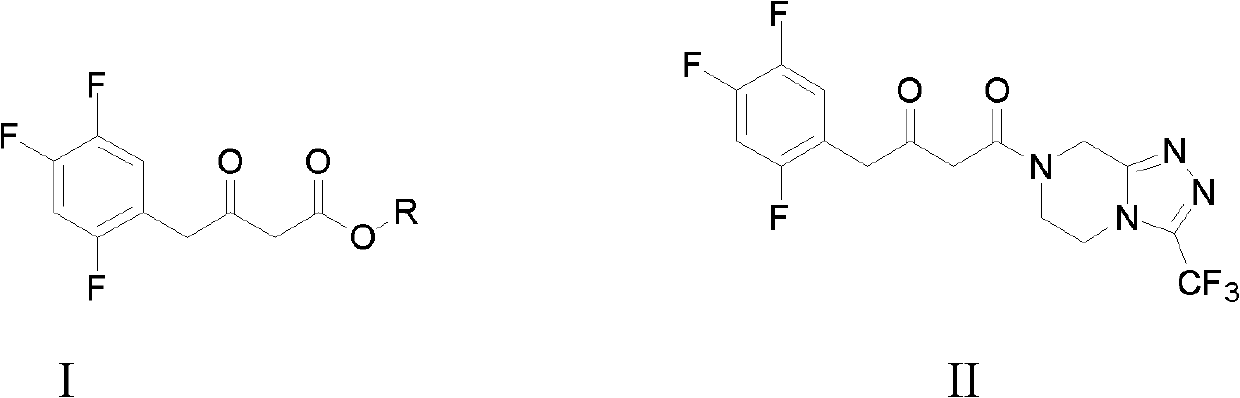
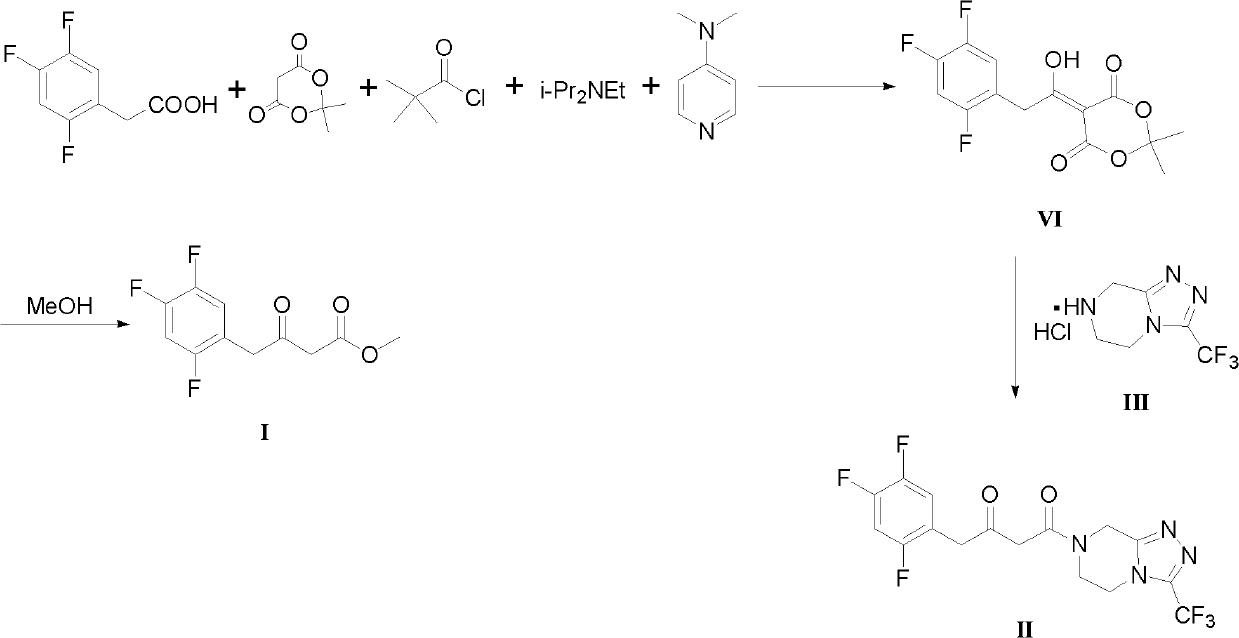
Preparation methods for sitagliptin intermediates
A technology for sitagliptin and an intermediate, which is applied in the field of pharmaceutical synthesis chemistry, can solve problems such as unfavorable large-scale production, complicated reaction process operations, etc., and achieves the effects of good industrialization prospects, simple operation and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Preparation of 3-carbonyl-4-(2,4,5-trifluorophenyl)-butyric acid methyl ester (I, R=Me)
[0036] Put magnesium chips (2.64g, 0.11mol) and diethyl ether (25mL) in a 250mL reaction flask, and slightly reflux for 10 minutes (to activate the magnesium powder). Cool down to room temperature, add a grain of iodine (initiator), and dropwise add a solution of methyl 2-bromoacetate (15.3 g, 0.10 mol) in ether 75 mL. After dropping, react at a temperature of 35°C for 1 hour to prepare the Grignard reagent for use.
[0037] Put 2,4,5-trifluorophenylacetonitrile (17.1 g, 0.10 mol) and diethyl ether (120 mL) in a 250 mL reaction flask, cool down to 0° C., and slowly add the above Grignard reagent dropwise. The temperature was controlled not to exceed 15°C, and the reaction was carried out at room temperature for 2 hours after the drop was completed. After the reaction is complete, lower the temperature to 0°C, add hydrochloric acid (2N, 75mL) dropwise under temperature c...
Embodiment 2
[0038] Example 2 Preparation of 3-carbonyl-4-(2,4,5-trifluorophenyl)-butyric acid ethyl ester (I, R=Et)
[0039] Put magnesium chips (3.46g, 0.14mol) and tetrahydrofuran (35mL) in a 250mL reaction flask, and stir at 40°C for 10 minutes under temperature control. After cooling down to room temperature, a grain of iodine was added, and a solution of ethyl 2-bromoacetate (21.7 g, 0.13 mol) in tetrahydrofuran (85 mL) was added dropwise. After dropping, react at a temperature of 40°C for 1 hour to prepare the Grignard reagent for use.
[0040] Put 2,4,5-trifluorophenylacetonitrile (22.2g, 0.13mol) and tetrahydrofuran (120mL) into a 500mL reaction flask, cool down to 0°C, and slowly add the above Grignard reagent dropwise. The temperature was controlled not to exceed 15°C, and the reaction was carried out at room temperature for 3 hours after the drop was completed. After the reaction is complete, lower the temperature to 0°C, add hydrochloric acid (2N, 100mL) dropwise at a temper...
Embodiment 3
[0041] Example 3 1-(3-trifluoromethyl-5,6-dihydro-8H-[1,2,4]triazole-[4,3-a]pyrazine-7-)-4-(2 , 4,5-trifluorophenyl)-1,3-butanedione (II) preparation
[0042] Bromoacetic acid (13.9g, 0.10mol), 3-(trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazine Hydrochloride (III, 22.8g, 0.10mol) and acetonitrile (100mL) were placed in a 250mL reaction flask, and thionyl chloride (17.7g, 0.15mol) was added dropwise at room temperature. The solvent was distilled off under reduced pressure, and the residue was dissolved in dichloromethane (150 mL), washed with water (50 mL), 5% sodium bicarbonate solution (50 mL) and saturated brine (50 mL) successively. Dry over anhydrous magnesium sulfate, filter with suction, and concentrate under reduced pressure to obtain intermediate IV (29.1 g, yield 93%).
[0043] Magnesium chips (2.43g, 0.10mol) and ether (50mL) were placed in a 250mL reaction flask, and a solution of intermediate IV (28.8g, 0.092mol) in ether (100mL) was added dropwi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com