Cannabidiol 2-butyrate and application thereof
A technology of cannabidiol and butyrate, which is applied in the preparation of carboxylic acid halides, preparations for toiletry, active ingredients of esters, etc., can solve the biological effects and drug potential that have not been disclosed, and cannot meet the needs of drug development, Biological role is not clear and other issues, to achieve the effect of high yield, mild conditions, low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1, the preparation of cannabidiol-2-butyrate
[0035] The synthetic route is shown in the following formula:
[0036]
[0037] Add 0.53g (5mmol) of butyryl chloride and 20mL of anhydrous acetonitrile into a 50mL three-neck flask, stir, add 1.57g (5mmol) of cannabidiol and 1mL of pyridine, stir to raise the temperature, reflux for 4 hours, and concentrate by rotary evaporation. Dissolved, washed with water, dried over anhydrous magnesium sulfate, rotary evaporated, separated and purified by column chromatography to obtain 1.39 g of a colorless liquid with a yield of 72.4%.
[0038] The H NMR spectrum of the product is characterized as follows:
[0039] 1 H NMR (300MHz, DMSO-d 6 )δ: 6.41(d, J=24.0Hz, 1H), 6.18(d, J=18.0Hz, 1H), 6.01(s, 1H), 5.05(d, J=21.0Hz, 1H), 4.46(d, J=21.0Hz, 2H), 3.83(d, J=9.0Hz, 1H), 3.03(t, J=9.0Hz, 1H), 2.66(s, 1H), 2.43-2.30(m, 3H), 2.08( s,1H),2.02-1.83(m,2H),1.67(s,2H),1.58(s,7H),1.48(d,J=6.0Hz,2H),1.30-1.26(m,5H),0.95 (t, ...
Embodiment 2
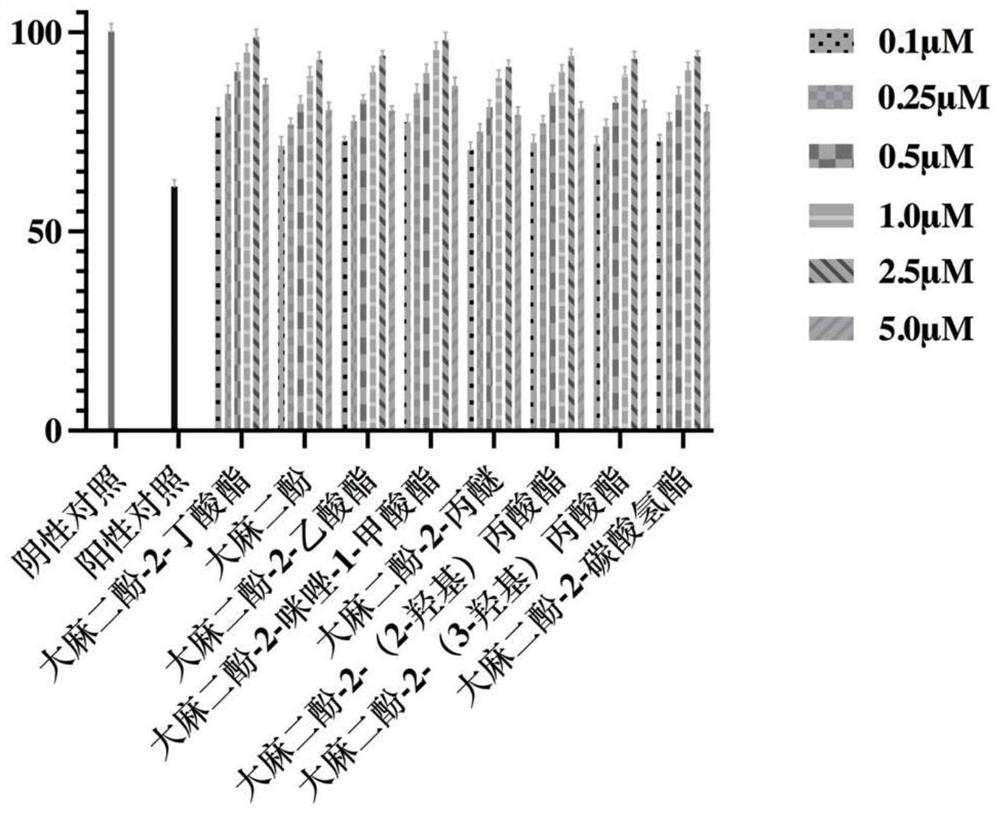
[0043] Example 2, Determination of the Nerve Cell Injury Protection of Cannabidiol-2-butyrate
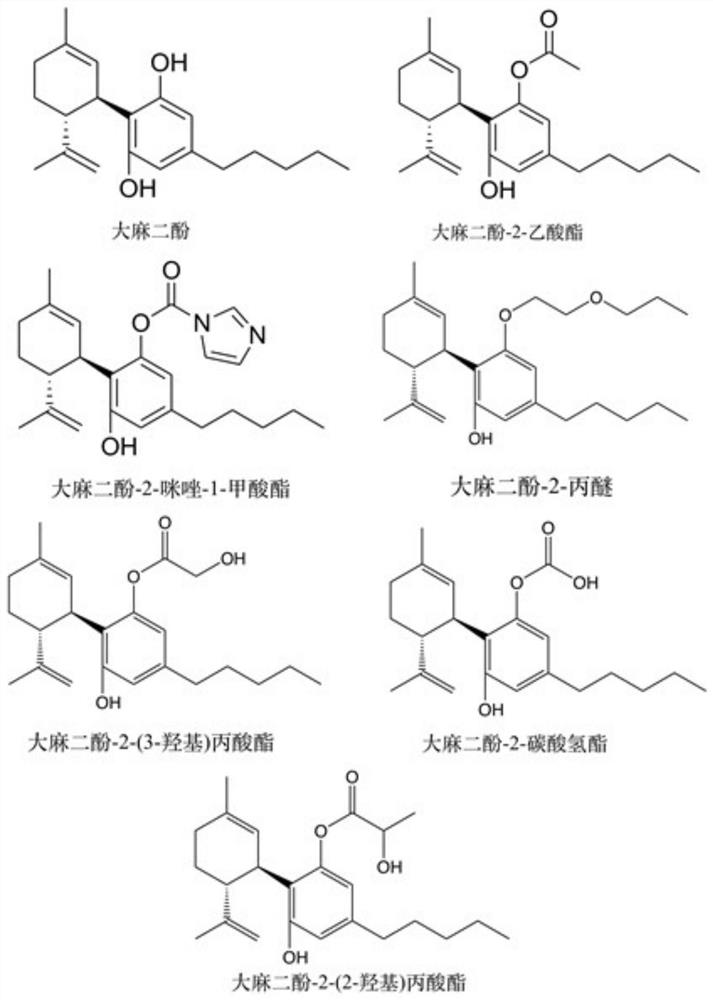
[0044] Under sterile conditions, the compound cannabidiol-2-butyrate and its analogs (cannabidiol, cannabidiol-2-acetate, cannabidiol-2-imidazole-1- Formate, cannabidiol-2-propyl ether, cannabidiol-2-(3-hydroxy)propionate, cannabidiol-2-bicarbonate, cannabidiol-2-(2-hydroxy)propionate acid ester) was dissolved in DMSO to prepare a 20mM stock solution. Glial cells BV2 were inoculated into a 96-well plate, respectively stimulated with glutamate and corticosterone to construct a cell injury model, and after 12 hours of culture, cannabidiol and the compound obtained in Example 1 were added to the medium and diluted to 5.0, 2.5, 1.0, 0.5, 0.25, 0.1 μM, set 5 duplicate wells for each group, continue to cultivate for 72 hours, add 20 μL of MTT reagent to each well, and continue to cultivate for 0.5 hours. Discard the MTT working solution, add 150 μl DMSO to each well, and shake gently on...
Embodiment 3
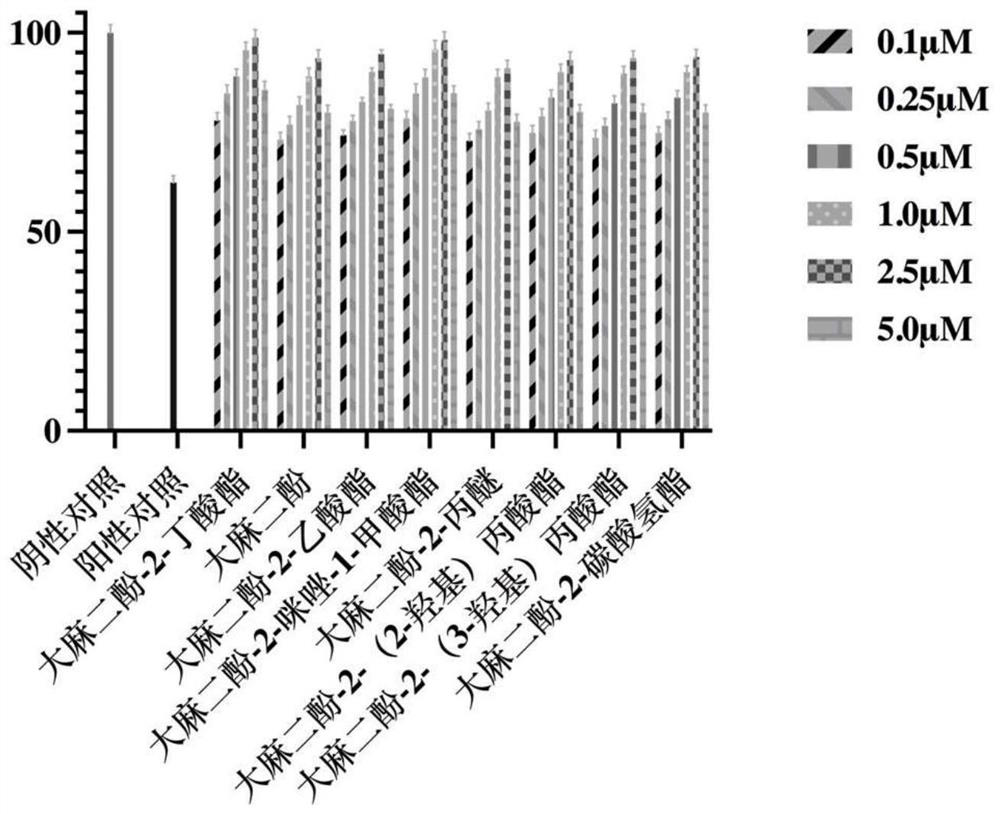
[0056] Embodiment 3, anti-breast cancer effect determination of cannabidiol-2-butyrate
[0057] Under sterile conditions, the compound cannabidiol-2-butyrate and its analogs (cannabidiol, cannabidiol-2-acetate, cannabidiol-2-imidazole-1- Formate, cannabidiol-2-propyl ether, cannabidiol-2-(2-hydroxy)propionate, cannabidiol-2-(3-hydroxy)propionate, cannabidiol-2-carbonic acid Hydrogen ester) was dissolved in DMSO to make a 20mM stock solution. Human breast cancer cells MDA-MB-231 were inoculated into 6-well plates, and three replicate wells were set for each group, and cultured for 12 hours. After the cells adhered to the wall, they were diluted into 5.0, 2.5, and 1.0 μM drug treatment groups by adding medium. Incubate for 24 hours. A normal control group was set up in the experiment. Digest the cells with EDTA-free trypsin, wash twice with PBS, resuspend the cells in 100 μL binding buffer, transfer to the flow tube, then add 5 μL Annexin V-FITC and 5 μL PI staining solution,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com