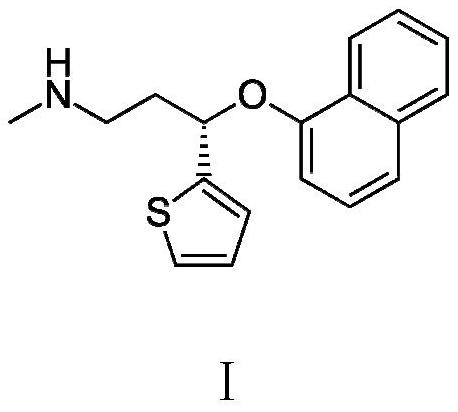
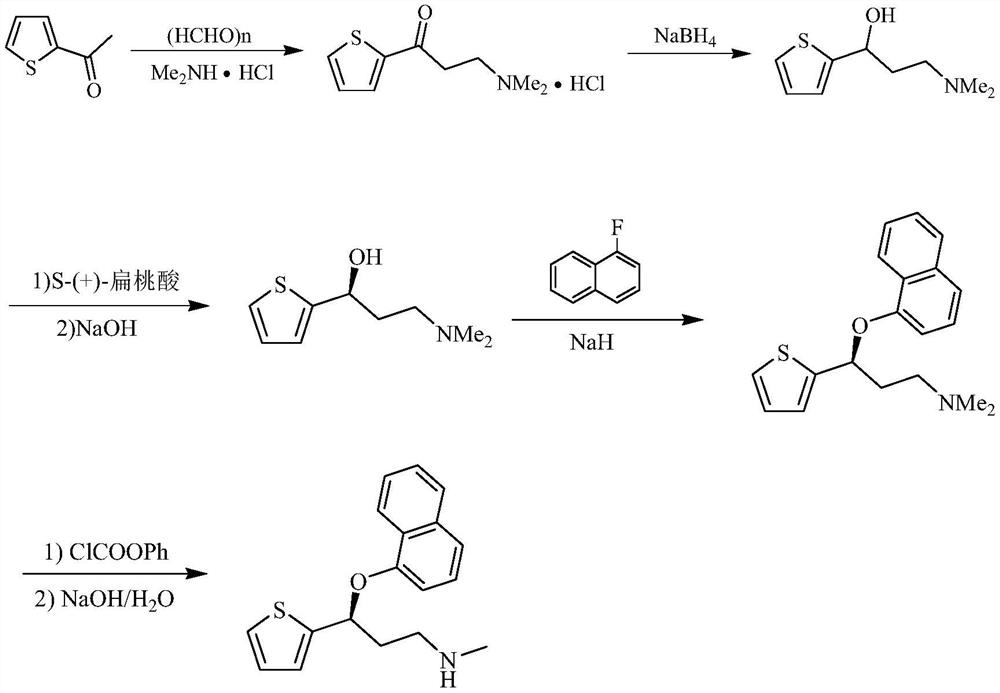
A kind of preparation method of duloxetine
A technology of duloxetine and compounds, which is applied in the field of duloxetine preparation, can solve the problems of difficulty in obtaining duloxetine with high optical purity, which is not conducive to the cost reduction of duloxetine, and the high price of 1-fluoronaphthalene. Achieve the effects of being conducive to green industrial production, classic reaction types, and high reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
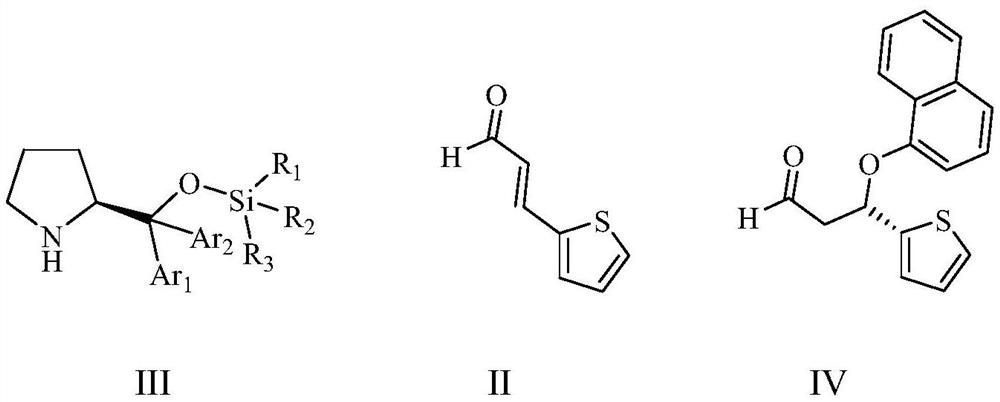
[0077] Example 1: Preparation of (S)-3-(1-naphthyloxy)-3-(2-thienyl)propanal (IV)
[0078] To a 2L four-necked flask equipped with a spherical condenser, stirring, thermometer and constant pressure dropping funnel, add 400 g of 1,2-dichloroethane, 138.2 g (1.0 mol) of 3-(2-thienyl)- 2-Acrolein (II), 26.0 g (0.08 mol) (S)-2-(1-trimethylsiloxy-1,1-diphenyl)methyltetrahydropyrrole (III1), 5-10 After stirring for half an hour, a mixed solution of 201.9 g (1.4 moles) of 1-naphthol and 400 g of 1,2-dichloroethane was added dropwise from a constant pressure dropping funnel, and the dropwise addition was completed in 3 hours. The reaction was stirred for 5 hours. Add 300 g of 20% aqueous sodium carbonate solution, stir at 20-25°C for half an hour, and separate layers. The obtained organic layer is washed once with 100 g of 5% hydrochloric acid and once with 100 g of saturated aqueous sodium chloride solution. The organic phase was dried with 25 grams of anhydrous sodium sulfate for ...
Embodiment 2
[0082] Example 2: Preparation of (S)-3-(1-naphthyloxy)-3-(2-thienyl)propanal (IV)
[0083] To a 2L four-necked flask equipped with a spherical condenser, stirring, thermometer and constant pressure dropping funnel, add 400 g of toluene, 138.2 g (1.0 mol) of 3-(2-thienyl)-2-propenal (II) , 32.5 g (0.1 mol) (S)-2-(1-trimethylsiloxy-1,1-diphenyl)methyltetrahydropyrrole (III1), after stirring at 10-15 °C for half an hour, the A mixed solution of 230.7 g (1.6 moles) of 1-naphthol and 600 g of toluene was added dropwise to a constant pressure dropping funnel, the dropwise addition was completed in 3 hours, and the reaction was stirred at 15-20° C. for 5 hours thereafter. Add 400 grams of 20% sodium carbonate aqueous solution, stir at 20-25°C for half an hour, and separate the layers. The obtained organic layer is washed once with 100 grams of 5% hydrochloric acid and once with 100 grams of saturated sodium chloride aqueous solution. The organic phase was dried with 25 grams of anhy...
Embodiment 3
[0084] Example 3: Preparation of (S)-3-(1-naphthyloxy)-3-(2-thienyl)propanal (IV)
[0085]To a 2L four-necked flask equipped with a spherical condenser, stirring, thermometer and constant pressure dropping funnel, add 400 g of 1,2-dichloroethane, 138.2 g (1.0 mol) of 3-(2-thienyl)- 2-Acrolein (II), 39.5 g (0.1 mol) (S)-2-(1-triethylsiloxy-1,1-di-p-methylphenyl)methyltetrahydropyrrole (III2), After stirring for half an hour at 5-10 °C, a mixed solution of 201.9 g (1.4 moles) of 1-naphthol and 400 g of 1,2-dichloroethane was added dropwise from a constant pressure dropping funnel, and the dropwise addition was completed for 3 hours, and thereafter 10 The reaction was stirred at -15°C for 5 hours. Add 300 g of 20% aqueous sodium carbonate solution, stir at 20-25°C for half an hour, and separate layers. The obtained organic layer is washed once with 100 g of 5% hydrochloric acid and once with 100 g of saturated aqueous sodium chloride solution. The organic phase was dried with 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com