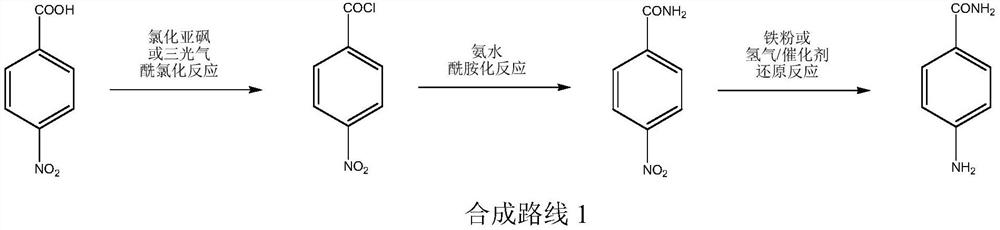
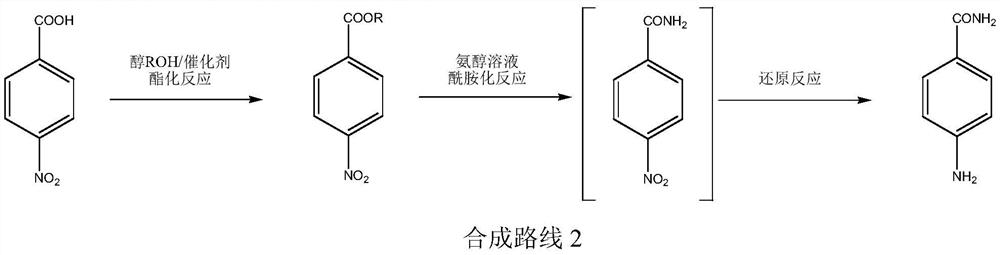
A kind of production technology of p-aminobenzamide
A technology of aminobenzamide and production process, which is applied in the field of production technology of p-aminobenzamide, can solve the problems of unfavorable green industrial production of aminobenzamide, high price of acyl chloride reagents, equipment corrosion, etc. Industrialized production, low cost and high total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Preparation of ethyl p-nitrobenzoate
[0036] In a 1000 ml four-neck flask connected with stirring, thermometer, water separator, and reflux condenser, 83.5 grams (0.5 moles) of p-nitrobenzoic acid, 250 grams of ethanol, 1.5 grams of p-toluenesulfonic acid, and 120 grams of toluene , 74.4 ° C (toluene-ethanol-water azeotropic) reflux with water for 7 hours, then cooled to 50-60 ° C, low vacuum and reduced pressure recovery ethanol and toluene (for the next batch of reactions), then high vacuum vacuum distillation ( 5-10mmHg) distilled 94.2 grams of ethyl p-nitrobenzoate, yield 96.5%, GC purity 99.9%.
[0037] (2) Preparation of p-aminobenzamide
[0038] In 500 milliliters of stainless steel autoclaves, add 39.0 grams (0.2 moles) ethyl p-nitrobenzoate prepared by step (1), 200 grams of 10% ammonia methanol solution, 0.3 grams of 5% palladium carbon catalyst, after nitrogen replacement three times, Pass in hydrogen, keep the hydrogen pressure at 0.2-0.3 MPa, and rea...
Embodiment 2
[0041] (1) Preparation of ethyl p-nitrobenzoate
[0042] In the 1000 milliliter four-neck flask that is connected with stirring, thermometer, water separator, reflux condenser, 83.5 grams (0.5 moles) p-nitrobenzoic acid, 230 grams of ethanol, 1.5 grams of solid super acid, 120 grams of normal hexane, 56 ℃ (n-hexane-ethanol-water azeotrope) was refluxed with water for 9 hours, then cooled to 40-50°C, low-vacuum and decompressed to recover n-hexane and ethanol (for the next batch of reactions), and then high-vacuum and reduced-pressure distillation ( 5-10mmHg) distilled 95.7 grams of ethyl p-nitrobenzoate, yield 98.2%, GC purity 99.8%.
[0043] (2) Preparation of p-aminobenzamide
[0044]Get 39.0 grams (0.2 moles) of ethyl p-nitrobenzoate prepared in step (1) and join in 500 milliliters of stainless steel autoclave, and add 200 grams of 10% ammonia ethanol solution, 3.5 grams of 50% Raney nickel catalyst, nitrogen displacement After three times, hydrogen gas was introduced to ...
Embodiment 3
[0046] (1) Preparation of ethyl p-nitrobenzoate
[0047] In a 1000 ml four-necked flask connected with stirring, thermometer, water separator, and reflux condenser, 75.0 grams (0.45 moles) of p-nitrobenzoic acid, 250 grams of ethanol, 1.5 grams of p-toluenesulfonic acid, and 150 grams of petroleum Ether, reflux at 65.5°C (petroleum ether-ethanol-water azeotrope) with water to react for 8 hours, then cool to 50-60°C, recover petroleum ether and ethanol (for the next batch reaction) under low vacuum and decompression, and then reduce Pressure distillation (5-10mmHg) distilled 86.5 grams of ethyl p-nitrobenzoate, yield 98.5%, GC purity 99.9%.
[0048] (2) Preparation of p-aminobenzamide
[0049] In 500 milliliters of stainless steel autoclaves, add 39.0 grams (0.2 moles) ethyl p-nitrobenzoate prepared by step (1), 200 grams of 10% ammonia methanol solution, 0.3 grams of 5% palladium carbon catalyst, after nitrogen replacement three times, Pass in hydrogen, keep the hydrogen pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com