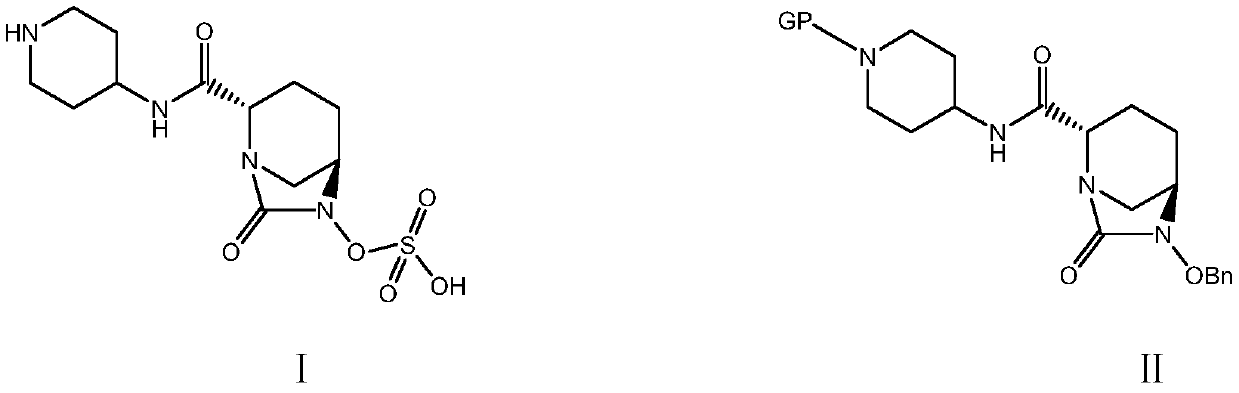
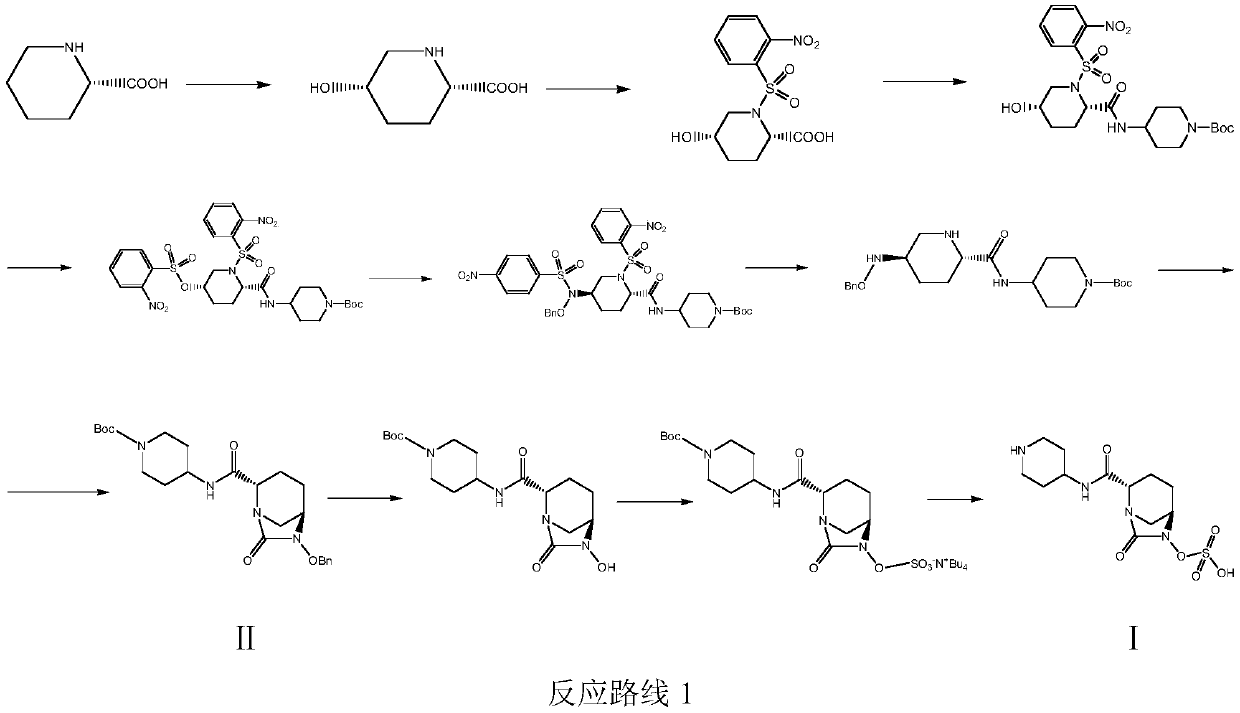
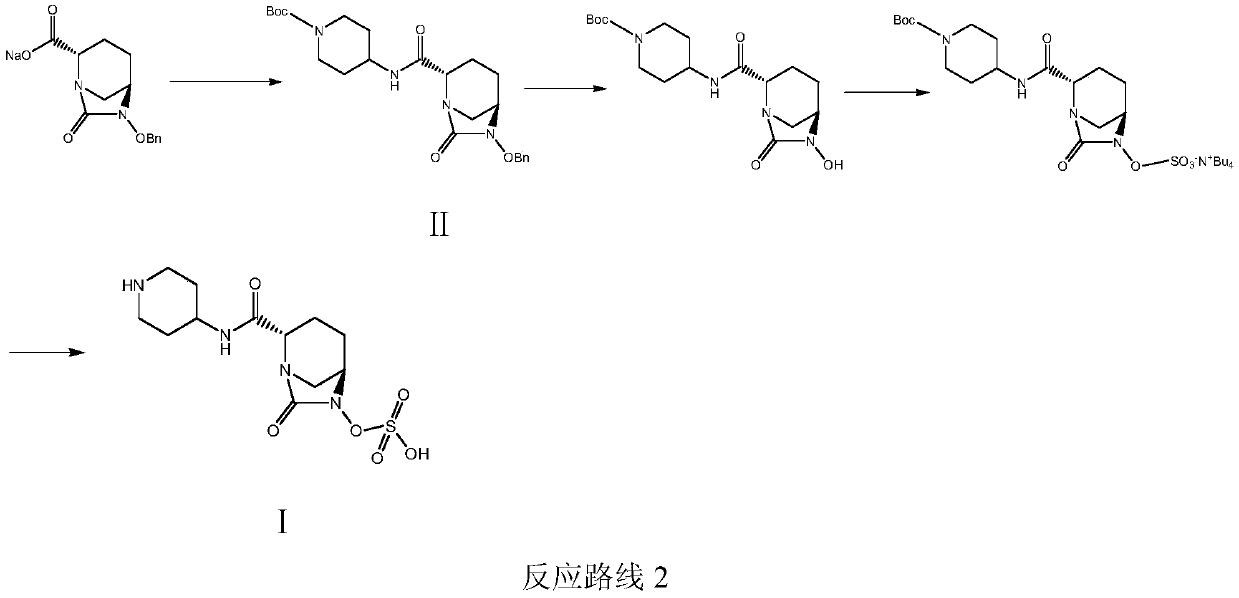
A kind of convenient preparation method of raylebactam intermediate
An intermediate and solid phosgene technology, which is applied in the field of pharmaceutical biochemical industry, can solve the problems of difficult acquisition, high price of sodium octane-2-formate, and no practical industrial value, etc., and achieves simple operation, easy control of reaction conditions, and wide variety of reagents little effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: (2S,5R)-N-(1-tert-butoxycarbonyl)piperidin-4-yl-6-benzyloxy-7-oxo-1,6-diazabicyclo[3.2. 1] Octane-2-carboxamide (Ⅱ 1 ) preparation
[0048] Add 200 grams of tetrahydrofuran, 12.5 grams (0.05 mole) (2S, 5R)-5-benzyloxyaminopiperidine-2-carboxylic acid, 50 grams of tri-n-butylamine, 0.1 gram of N,N-dimethylformamide, cooled, at -10-0°C, dropwise added a mixed solution of 23.8g (0.08 moles) of solid phosgene and 80g of tetrahydrofuran, and stirred at 10-20°C for 4 hours. Between 10-20°C, add a mixed solution of 12.0 g (0.06 mole) 1-tert-butoxycarbonyl-4-aminopiperidine and 40 g of tetrahydrofuran, stir and react for 3 hours between 15-20°C, pour the reaction liquid into 300 g of ice-water mixture, separated into layers, and the aqueous layer was extracted twice with dichloromethane, 50 g each time. The combined organic phases were washed twice with saturated sodium chloride solution, each time 20 grams, and after the solvent was recovered from the gained orga...
Embodiment 2
[0051] Example 2: (2S,5R)-N-(1-tert-butoxycarbonyl)piperidin-4-yl-6-benzyloxy-7-oxo-1,6-diazabicyclo[3.2. 1] Octane-2-carboxamide (Ⅱ 1 ) preparation
[0052] Add 200 grams of dichloromethane, 12.5 grams (0.05 moles) of (2S, 5R)-5-benzyloxyaminopiperidine-2-carboxylic acid, 50 grams of diisopropyl Ethylamine, 0.1 g of N, N-dimethylformamide, cooled, at -5-0 ° C, dropwise add a mixed solution of 23.8 g (0.08 mole) of solid phosgene and 80 g of dichloromethane, dropwise 15-20 The reaction was stirred at °C for 4 hours. Between 15-20°C, add a mixed solution of 14.0 grams (0.07 moles) of 1-tert-butoxycarbonyl-4-aminopiperidine and 40 grams of dichloromethane, stir and react for 3 hours between 15-20°C, and the reaction The liquid was poured into 300 g of ice-water mixture, the layers were separated, and the aqueous layer was extracted twice with 50 g of dichloromethane each time. The combined organic phases were washed twice with saturated sodium chloride solution, 20 grams eac...
Embodiment 3
[0053] Example 3: (2S,5R)-N-(1-tert-butoxycarbonyl)piperidin-4-yl-6-benzyloxy-7-oxo-1,6-diazabicyclo[3.2. 1] Octane-2-carboxamide (Ⅱ 1 ) preparation
[0054] Add 200 grams of THF, 12.5 grams (0.05 moles) of (2S, 5R)-5-benzyloxyaminopiperidine-2-carboxylic acid, 60 grams of diisopropylethylamine in a 500 milliliter four-necked flask equipped with stirring and a thermometer. , 0.1 g of N,N-dimethylformamide, cooled, and at -10-0°C, dropwise added a mixed solution of 25.0 g (0.13 moles) of diphosgene and 80 g of tetrahydrofuran, and stirred at 10-20°C for 5 Hour. Between 10-20°C, add a mixed solution of 14.0 grams (0.07 moles) of 1-tert-butoxycarbonyl-4-aminopiperidine and 40 grams of tetrahydrofuran, stir and react for 5 hours between 15-20°C, pour the reaction liquid into 300 g of ice-water mixture, separated into layers, and the aqueous layer was extracted twice with dichloromethane, 50 g each time. The combined organic phases were washed twice with saturated sodium chlori...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com