3-aminomethyl tetrahydrofuran preparation method
A technology of aminomethyltetrahydrofuran and tetrahydrofuran, which is applied in the field of pesticide chemistry, can solve the problems of expensive catalysts, poor atom economy, difficult recycling and reuse, etc., and achieve the effects of low cost, avoiding hydrogenation reduction, and high product yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
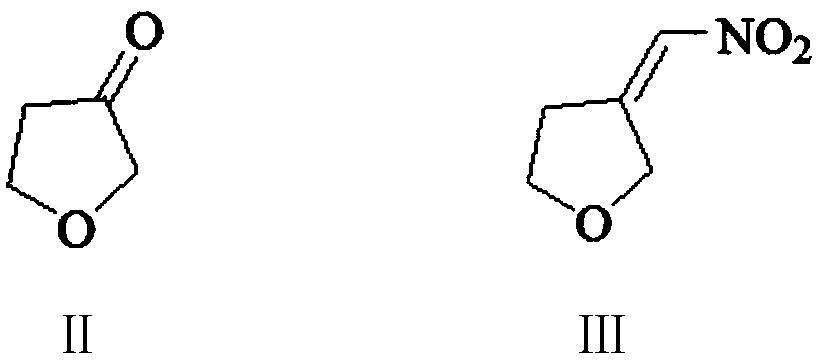
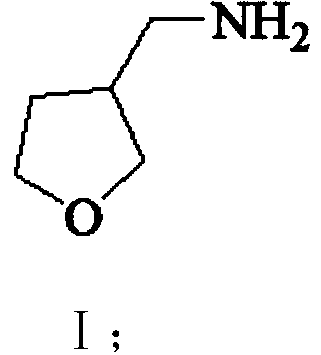
[0056] Embodiment 1: Preparation of 3-nitromethylenetetrahydrofuran (Ⅲ)
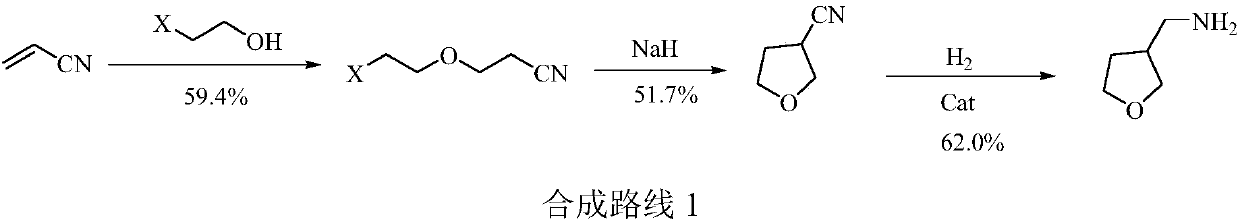
[0057] In the 500 milliliter four-necked flask that is connected with stirring, thermometer, reflux condenser and dropping funnel, add 180 gram methanol, 2.0 gram 27wt% sodium methylate methanol solution, 25.6 gram (0.42 moles) nitromethane, 20 to 25 ℃ In between, a mixture of 34.4 g (0.4 mol) of tetrahydrofuran-3-one (II) and 50 g of methanol was added dropwise, and it took about 2 hours to complete the dropwise addition. Thereafter, the reaction was stirred at 25-30° C. for 4 hours. Cool to 0 to 5°C, add 100 grams of water, 100 grams of dichloromethane, adjust the pH of the system to 6.5-7.5 with 30 wt% hydrochloric acid, separate layers, and extract the aqueous layer 3 times with dichloromethane, each with 30 grams of dichloromethane methane, combined the organic phases, distilled and recovered dichloromethane to obtain 49.9 g of 3-nitromethylenetetrahydrofuran (III), with a yield of 96.7% and a gas p...
Embodiment 2
[0058] Embodiment 2: Preparation of 3-nitromethylenetetrahydrofuran (Ⅲ)
[0059] In the 500 milliliter four-necked flask that is connected with stirring, thermometer, reflux condenser and dropping funnel, add 180 gram methanol, 1.0 gram sodium hydroxide, 26.2 gram (0.43 mole) nitromethane, between 25 to 30 ℃, A mixture of 34.4 g (0.4 moles) of tetrahydrofuran-3-one (II) and 50 g of methanol was added dropwise for about 2 hours. After that, the reaction was stirred at 30 to 35° C. for 4 hours. Cool to 0 to 5°C, add 100 grams of water, 100 grams of dichloromethane, adjust the pH of the system to 6.5-7.5 with 30 wt% hydrochloric acid, separate layers, and extract the aqueous layer 3 times with dichloromethane, each with 30 grams of dichloromethane methane, combined the organic phases, distilled and recovered dichloromethane to obtain 48.3 g of 3-nitromethylenetetrahydrofuran (III), with a yield of 93.6% and a gas phase purity of 99.4%.
Embodiment 3
[0060] Embodiment 3: Preparation of 3-aminomethyltetrahydrofuran (I)
[0061] Add 200 grams of methanol, 25.8 grams (0.2 moles) of 3-nitromethylenetetrahydrofuran (Ⅲ) prepared in Example 1 in a 500 milliliter stainless steel autoclave, 0.5 grams of 5wt% palladium carbon catalyst, nitrogen replacement three times, pass into Hydrogen, keep the system pressure at 0.3-0.4MPa, react at 30-35°C for 4 hours. Nitrogen was replaced three times, the palladium carbon was removed by filtration, the filter cake was washed twice with methanol, 30 grams of methanol each time, and the filtrates were combined. The filtrate was distilled to recover the solvent, and then distilled under reduced pressure (85-95°C / 2mmHg) to obtain 19.6 g of colorless transparent liquid 3-aminomethyltetrahydrofuran (I), with a yield of 97.2% and a gas phase purity of 99.7%.
[0062] The NMR data of the resulting product are as follows:
[0063] 1 HNMR (400MHz, CDCl 3 ):δppm
[0064] 3.82-3.86(m,2H),3.71-3.75(t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com