Preparation method of vitamin A ester intermediate C15 and vitamin A ester
A technology for vitamins and intermediates, which is applied in the field of preparation of vitamin A ester intermediate C15 and vitamin A ester, can solve the problems of complicated post-processing, low purity of vitamin A palmitate, unenvironmental protection and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
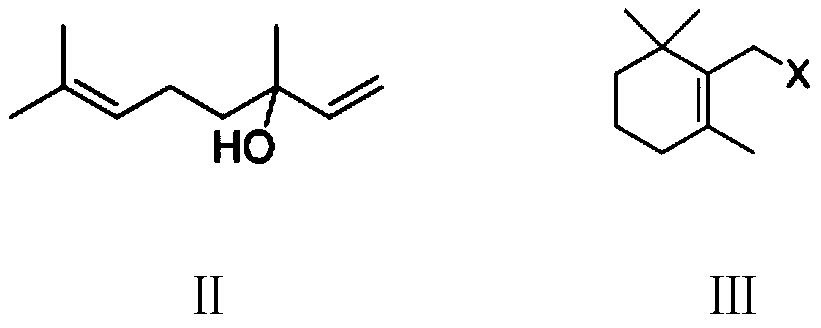
[0122] Example 1: 2,6,6-trimethyl-2-chloromethylcyclohexene (Ⅲ 1 ) preparation
[0123] Add 500 g of 1,2-dichloroethane, 154.0 g (1.0 mol) of 3,7-di Methyl-3-hydroxy-1,6-octadiene, 131.0 g (1.1 moles) of thionyl chloride, kept between 40°C and 45°C, stirred for 3 hours, cooled to 10-15°C to obtain chlorinated The reaction liquid was transferred to a constant pressure dropping funnel and set aside. In another 1000 ml four-necked flask equipped with stirring, thermometer, reflux condenser and constant pressure dropping funnel, add 100 g of 1,2-dichloroethane, 5.0 g of 98% concentrated sulfuric acid, and keep it at 70°C to 75°C. Between ℃, add the obtained chlorination reaction liquid dropwise, and the dropwise addition is completed in 2 hours. After that, stir and react at 70℃~75℃ for 3 hours, cool to 10-15℃, add 100 grams of water, stand to separate layers, and distill the organic phase After recovering the solvent, 171.0 grams of colorless liquid 2,6,6-trimethyl-2-chloromet...
Embodiment 2
[0124] Example 2: 2,6,6-trimethyl-2-chloromethylcyclohexene (Ⅲ 1 ) preparation
[0125] Under nitrogen protection, add 500 g of dichloromethane, 154.0 g (1.0 mole) of 3,7-dimethyl Base-3-hydroxyl-1,6-octadiene, 135.6 g (1.3 moles) of 35% hydrochloric acid aqueous solution, kept between 30°C and 35°C, stirred and reacted for 2 hours, cooled to 10-15°C, and separated into layers to obtain The organic phase is the chlorination reaction liquid, which is transferred to a constant-pressure dropping funnel for later use. In another 1000 ml four-necked flask equipped with stirring, thermometer, reflux condenser and constant pressure dropping funnel, add 100 g of dichloromethane, 5.0 g of 98% concentrated sulfuric acid, keep between 35°C and 40°C, drop Add the obtained chlorinated reaction liquid, drop it in 2 hours, after that, stir and react at 35°C to 40°C for 4 hours, cool to 10-15°C, add 100 grams of water, stand to separate layers, and distill the organic phase to recover the s...
Embodiment 3
[0126] Example 3: 2,6,6-trimethyl-2-bromomethylcyclohexene (Ⅲ 2 ) preparation
[0127] Under nitrogen protection, add 500 g of dichloromethane, 154.0 g (1.0 mole) of 3,7-dimethyl Base-3-hydroxy-1,6-octadiene, 220.0 g (1.1 mol) 40% hydrobromic acid aqueous solution, kept between 30°C and 35°C, stirred for 2 hours, cooled to 10-15°C, separated , the organic phase was obtained as a bromination reaction liquid, which was transferred to a constant pressure dropping funnel for subsequent use. In another 1000 ml four-necked flask equipped with stirring, thermometer, reflux condenser and constant pressure dropping funnel, add 100 g of dichloromethane, 5.0 g of 98% concentrated sulfuric acid, keep between 35°C and 40°C, drop Add the obtained bromination reaction liquid, and drop it in 2 hours. After that, stir and react at 35°C to 40°C for 3 hours, cool to 10-15°C, add 100 grams of water, let stand to separate layers, and distill the organic phase to recover the solvent to obtain 19...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com