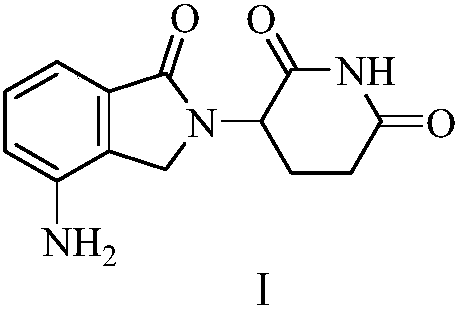
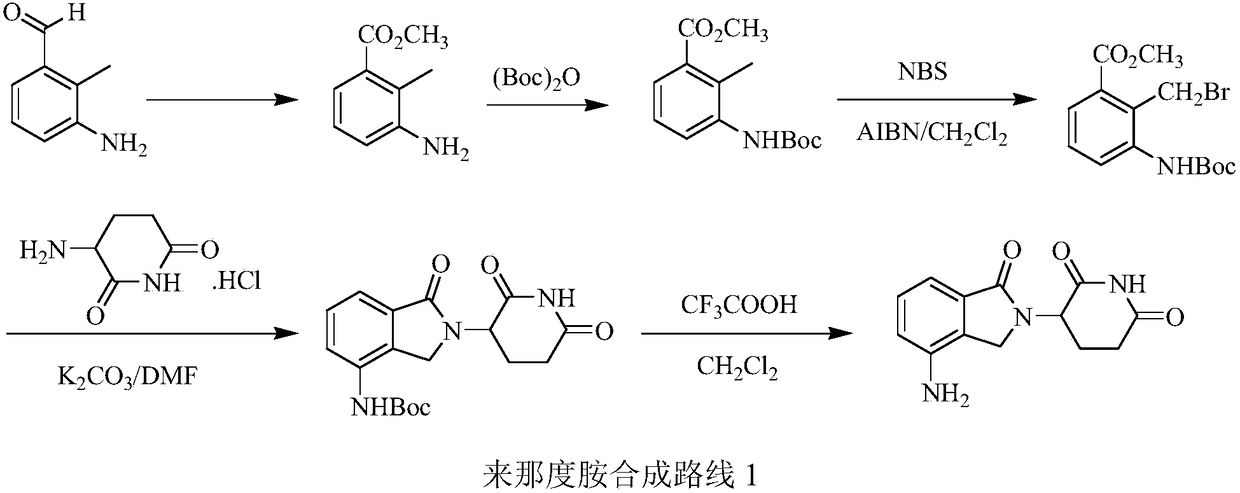
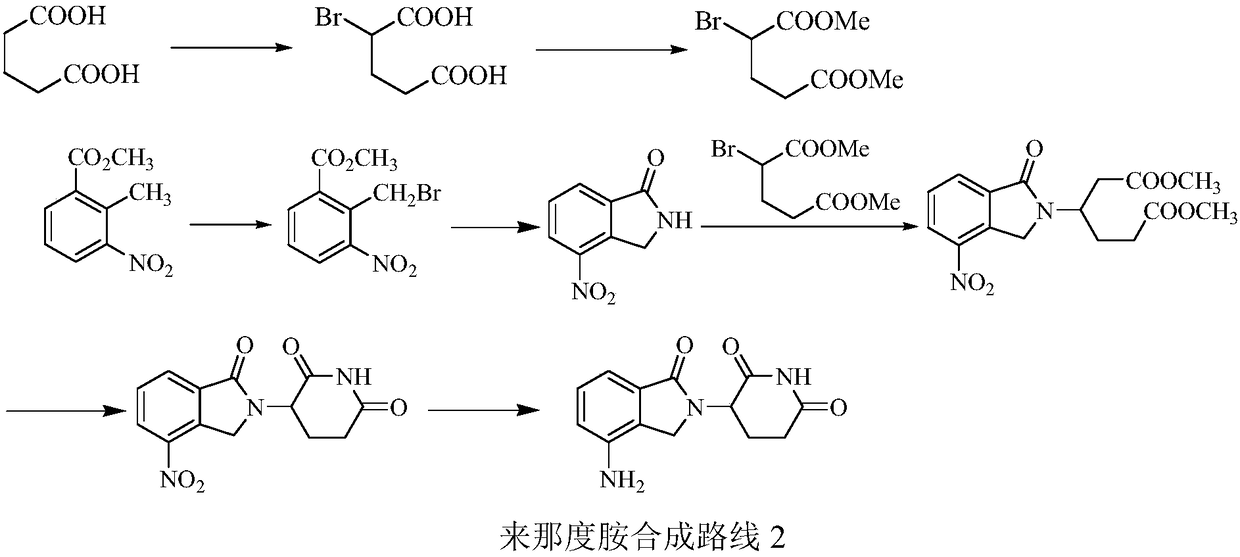
Green production method of low-cost lenalidomide
A lenalidomide and green production technology, applied in the field of medicinal chemistry, can solve problems such as unfavorable safety production and environmental protection, difficulty in realizing large-scale production, difficulty in large-scale production, etc., and achieve cost reduction, mild process conditions and low price Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1: the preparation of 2-chloro-4-nitro n-butyraldehyde
[0052] Under nitrogen protection, into a 500 ml four-necked flask connected with stirring, a thermometer and a reflux condenser, add 90.5 g (1.0 mol) of 2-chloroacrolein, 91.5 g (1.5) of nitromethane, 2.0 g of DBU (1, 8-diazabicycloundec-7-ene), stirred and reacted at 90~95°C for 8 hours, after the excess nitromethane was recovered by atmospheric distillation, 144.3 grams of product 2-chloro-4-nitro was obtained by vacuum distillation N-butyraldehyde, yield 95.2%, gas phase purity 99.6%.
Embodiment 2
[0053] Embodiment 2: Preparation of 2-bromo-4-nitro-n-butyraldehyde
[0054] Under nitrogen protection, add 135 g (1.0 mol) of 2-bromoacrolein, 91.5 g (1.5) of nitromethane, 2.0 g of DBU, 95 to The reaction was stirred at 100° C. for 4 hours. After the excess nitromethane was recovered by atmospheric distillation, 175.5 g of product 2-bromo-4-nitro-n-butyraldehyde was obtained by distillation under reduced pressure, with a yield of 89.5% and a gas phase purity of 99.3%.
Embodiment 3
[0055] Example 3: Preparation of 3-(7-nitro-3-oxo-1H-isoindol-2-yl)piperidine-2,6-dione (formula VI)
[0056] Under nitrogen protection, add 120 g of N,N-dimethylformamide, 12.8 g (0.1 mol) of 3-aminopiperidine-2,6 - diketone, 16.5 grams (0.11 moles) of methyl 1-chloroacetoacetate, 40 grams of potassium carbonate, stirred and reacted at 40 to 45 ° C for 4 hours, then stirred and reacted at 90 to 95 ° C for 3 hours, and steamed out the methanol generated by the amidation reaction ; Cool to 40°C, add 16.5 (0.11 mole) 2-chloro-4-nitro-n-butyraldehyde, stir and react at 40-45°C for 5 hours, filter, and wash the filter cake with 40 grams of N,N-dimethylformamide , combined the filtrates, recovered N,N-dimethylformamide by distillation, added 90 grams of isopropanol, and recrystallized to obtain 27.1 grams of 3-(7-nitro-3-oxo-1H-isoindole-2- base) piperidine-2,6-dione, the yield is 93.7%, and the liquid phase purity is 99.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com