Synthesis method of chickpea dentin A
A synthesis method and bean curd technology, applied in the direction of organic chemistry and the like, can solve the problems of easy agglomeration of the reaction system, incomplete reaction and high risk, and achieve the effects of saving synthesis time, prolonging service life and reducing reaction temperature.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] A synthetic method for biochanin A, comprising the following steps:
[0043] 1) condensation
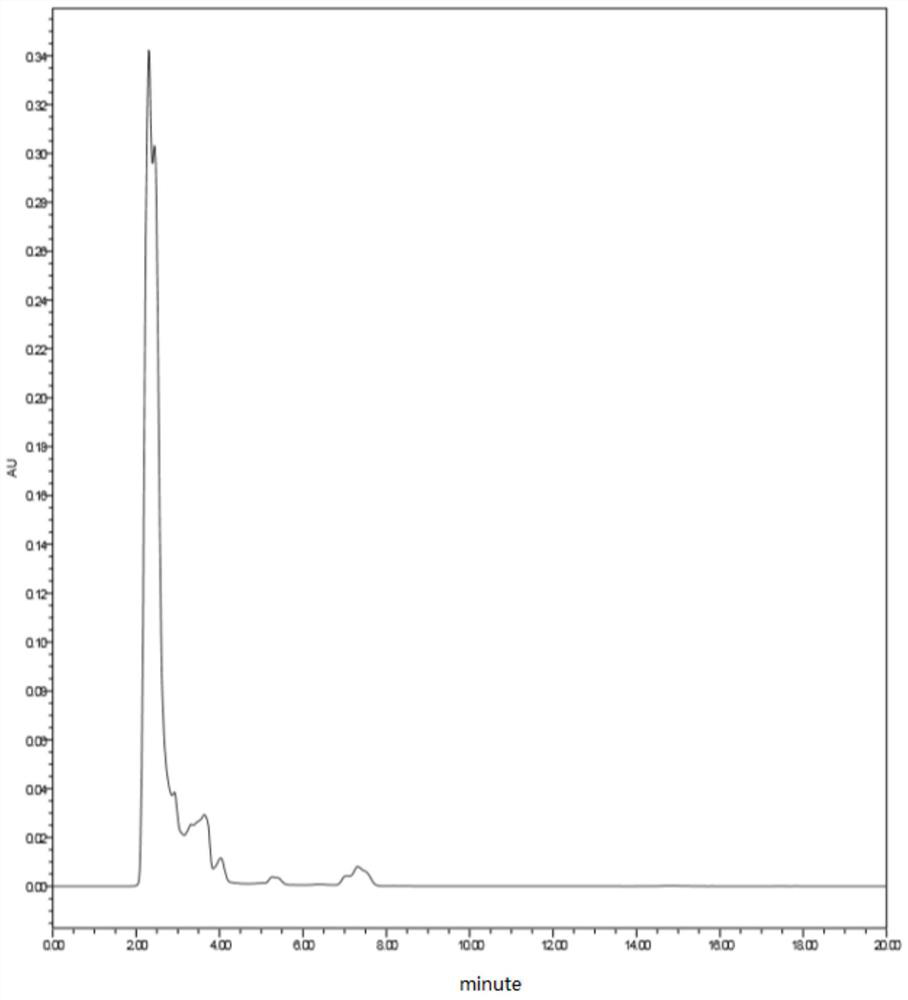
[0044] Mix 43.8g of p-methoxyphenylacetonitrile and 200g of ethyl acetate, continuously feed dry hydrogen chloride gas into the mixture at 5-10°C, and stir for reaction; ventilate for 4 hours to make the system saturated with hydrogen chloride, then add 38g of hydrogen chloride without Phloroglucinol water, after continuing to ventilate for 12 hours, when the liquid-phase central control monitors the content of anhydrous phloroglucinol below 1%, the reaction ends and a condensation product is obtained; wherein, for the condensation reaction mid-control liquid phase spectrum, see figure 1 ;
[0045] 2) Hydrolysis
[0046] The condensation product obtained in step 1) was distilled under reduced pressure, ethyl acetate was distilled off, and then 400 g of water was added to conduct a hydrolysis reaction at 70° C. for about 8 hours. After the reaction was completed, it was coole...
Embodiment 2
[0052] A synthetic method for biochanin A, comprising the following steps:
[0053] 1) condensation
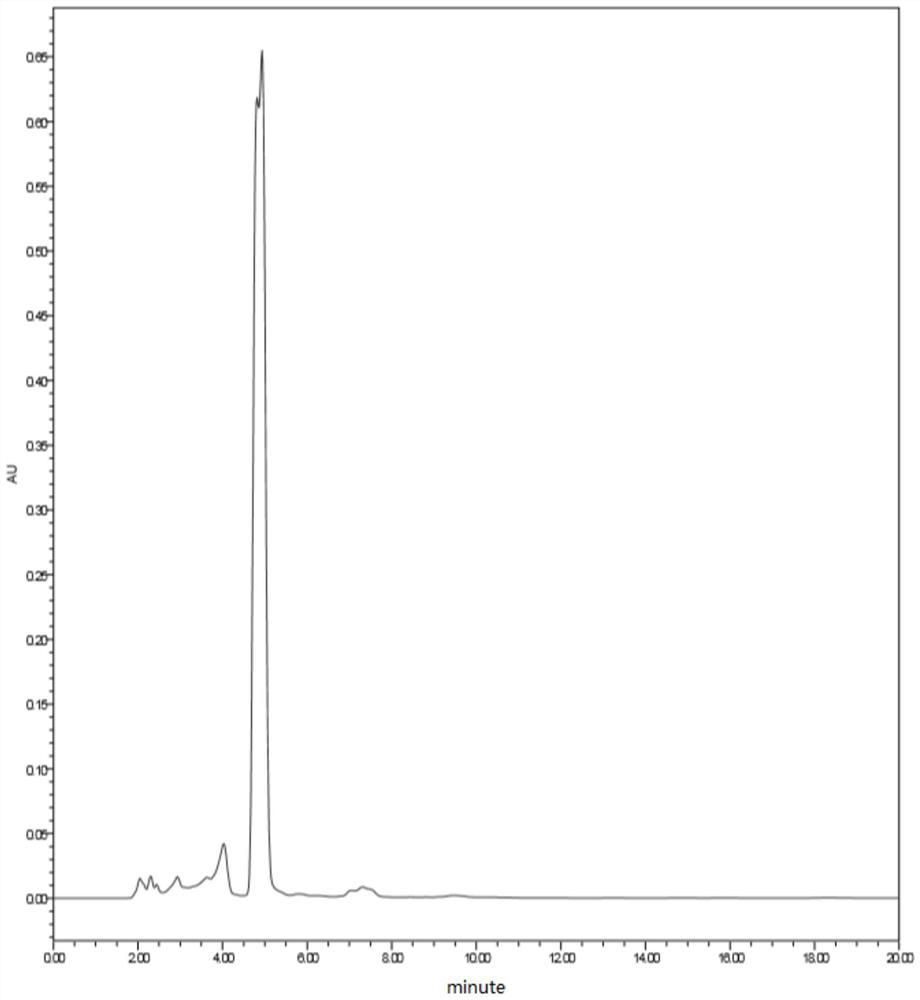
[0054] Mix 21.9g of p-methoxyphenylacetonitrile and 100g of ethyl acetate, continuously feed dry hydrogen chloride gas into the mixture at 5-10°C, and stir for reaction; ventilate for 4 hours to make the system saturated with hydrogen chloride, then add 19g of hydrogen chloride without Phloroglucinol water, after continuing to ventilate for 12 hours, when the liquid-phase central control monitors the content of anhydrous phloroglucinol below 1%, the reaction ends and a condensation product is obtained; wherein, for the condensation reaction mid-control liquid phase spectrum, see Figure 4 ;
[0055] 2) Hydrolysis
[0056] Distill the condensation product obtained in step 1) under reduced pressure, distill off the ethyl acetate, then add 200 g of water to carry out the hydrolysis reaction at 72°C for about 8 hours, after the reaction, cool to room temperature, suction filter,...
Embodiment 3
[0062] A synthetic method for biochanin A, comprising the following steps:
[0063] 1) condensation
[0064] Mix 44kg of p-methoxyphenylacetonitrile and 200kg of ethyl acetate, continuously feed dry hydrogen chloride gas into the mixture at 5-10°C, and stir for reaction; ventilate for 4 hours to make the system saturated with hydrogen chloride, then add 38kg of anhydrous For phloroglucinol, when the anhydrous phloroglucinol content is lower than 1% in liquid-phase central control monitoring after continuing to ventilate for 12 hours, the reaction ends and a condensation product is obtained;
[0065] 2) Hydrolysis
[0066] The condensation product obtained in step 1) was distilled under reduced pressure, ethyl acetate was distilled off, and then 400 kg of water was added to conduct a hydrolysis reaction at 70° C. for about 8 hours. After the reaction was completed, it was cooled to room temperature and suction filtered, and dried to obtain 63 kg of the intermediate;
[0067] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com