Method for continuously preparing voriconazole intermediate 2-fluoro-3-oxopentanoic acid ethyl ester
A technology of ethyl oxyvalerate and voriconazole, which is applied in the separation/purification of carboxylate, preparation of carboxylate, chemical instruments and methods, etc., can solve the problem that the purity of ethyl 2-fluoro-3-oxyvalerate is difficult to further improve. Improvements, technical difficulties, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
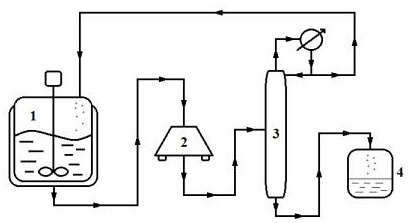
[0024] The reaction product is qualitatively and quantitatively detected by high-performance liquid chromatography: octadecylsilane bonded silica gel is used as a filler (4.6mm×150mm, 3μm or a chromatographic column with equivalent performance), and 0.03mol / L disodium hydrogen phosphate solution (Adjust the pH value to 6.5 with phosphoric acid)-acetonitrile (55:45) as the mobile phase; the detection wavelength is 230nm, and the injection volume is 20 μl.
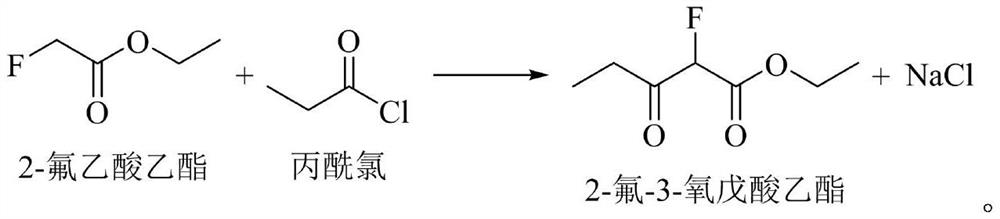
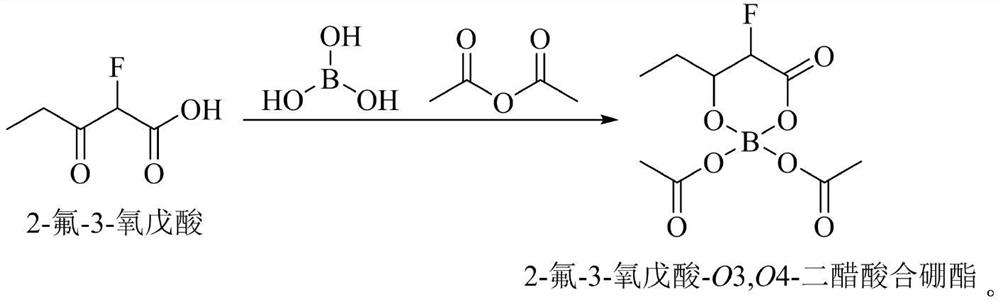
[0025] Using tetrahydrofuran as the reaction solvent, propionyl chloride and 2-fluoro-ethyl acetate are pumped into the reaction kettle at a molar ratio of 5:1; sodium hydrogen and 2-fluoro-ethyl acetate are pumped into the reaction kettle at a molar ratio of 1:1. Heat to the reaction temperature of 35°C, stir vigorously for 10 h, add boric acid and acetic anhydride, so that the molar ratio of boric acid, acetic anhydride and 2-fluoro-ethyl acetate is 1:1:5. The reaction solution is pumped from the bottom of the reaction ket...
Embodiment 2
[0027] Reaction product qualitative and quantitative detection method and operation are all the same as in Example 1, and the implementation steps of changing the reactant molar proportion and each operating parameter are as follows:
[0028] With tetrahydrofuran as the reaction solvent, propionyl chloride and 2-fluoro-ethyl acetate are pumped into the reaction kettle in a molar ratio of 4:1; sodium hydrogen and 2-fluoro-ethyl acetate are pumped into the reaction kettle in a molar ratio of 3:1. Heat to the reaction temperature of 60°C, stir vigorously for 3 hours, add boric acid and acetic anhydride, so that the molar ratio of boric acid, acetic anhydride and 2-fluoro-ethyl acetate is 1:1:8. The reaction solution is pumped from the bottom of the reaction kettle into a centrifuge to remove the generated sodium chloride. The filtrate is continuously pumped into the middle of the rectification tower, heated and rectified to 85°C, and the product is separated, the light component ...
Embodiment 3
[0030] Reaction product qualitative and quantitative detection method and operation are all the same as in Example 1, and the implementation steps of changing the reactant molar proportion and each operating parameter are as follows:
[0031] With tetrahydrofuran as the reaction solvent, propionyl chloride and 2-fluoro-ethyl acetate are pumped into the reaction kettle at a molar ratio of 3:1; sodium hydrogen and 2-fluoro-ethyl acetate are pumped into the reaction kettle at a molar ratio of 6:1. Heat to the reaction temperature of 20°C, stir vigorously for 1 h, add boric acid and acetic anhydride, so that the molar ratio of boric acid, acetic anhydride and 2-fluoro-ethyl acetate is 1:1:13. The reaction solution is pumped from the bottom of the reaction kettle into a centrifuge to remove the generated sodium chloride. The filtrate is continuously pumped into the middle of the rectification tower, heated and rectified to 100°C, and the product is separated, the light component pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com