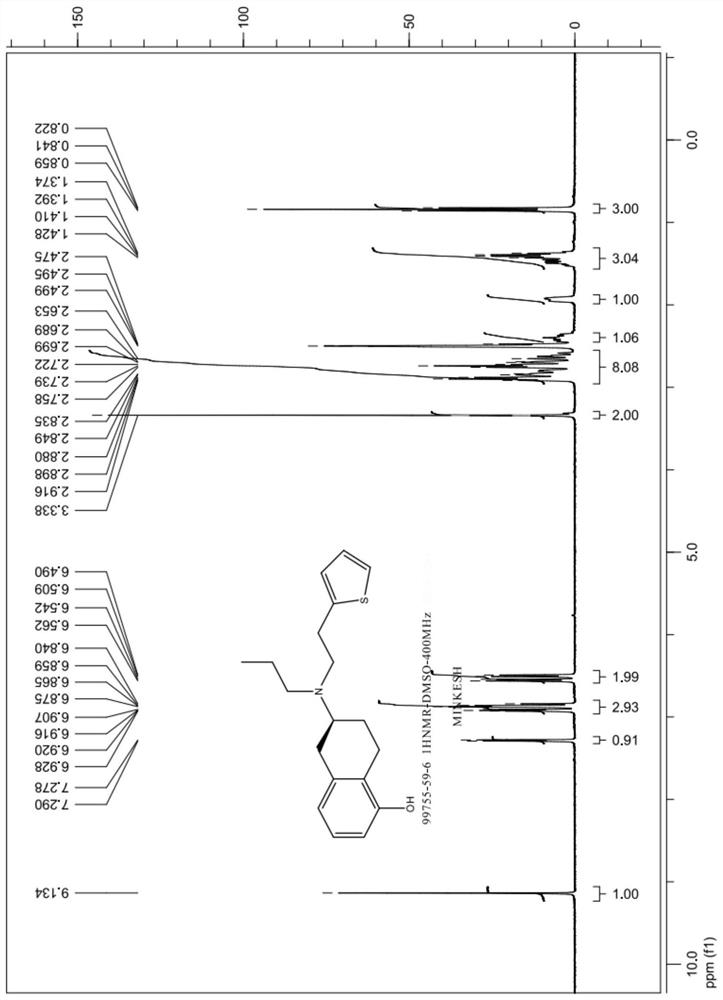
A kind of preparation method of rotigotine
A technology of methoxyl and tetrahydronaphthalene, which is applied in the field of pharmaceutical preparation, can solve the problems of large consumption of raw materials, corrosion of equipment, difficulty in disassembly, etc., and achieve the effects of reducing loss, easy operation and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
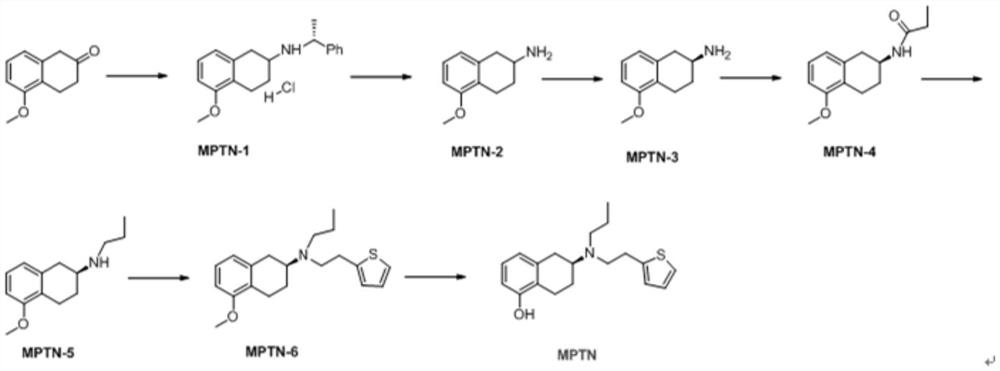
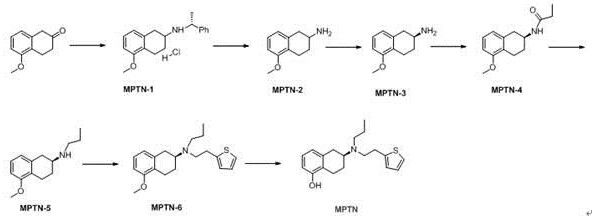
[0025] The invention provides a kind of preparation method of rotigotine, comprises the steps:
[0026] S1 Dissolve 5-methoxyl-2-tetralone in an organic solvent, add R-α-methylbenzylamine dropwise to react, after the end, add a reducing agent to process to obtain 5-methoxyl-N(( R)-1-phenethyl)-1,2,3,4-tetrahydronaphthalene-2-amine hydrochloride;
[0027] S2 debenzylation reaction of 5-methoxy-N((R)-1-phenylethyl)-1,2,3,4-tetrahydronaphthalene-2-amine hydrochloride, after the end, in the base Reactive conditions are processed to obtain 5-methoxy-1,2,3,4-tetrahydronaphthalene-2-amine;
[0028] S3 Dissolve 5-methoxy-1,2,3,4-tetrahydronaphthalene-2-amine in an organic solvent, add S-mandelic acid solution dropwise, after the dropwise addition, suction filter after reaction to obtain a solid, After separation, (S)-5-methoxy-1,2,3,4-tetrahydronaphthalene-2-amine was obtained;
[0029] S4 reacts (S)-5-methoxy-1,2,3,4-tetrahydronaphthalene-2-amine with propionyl chloride reagent to...
Embodiment 1
[0055] Example 1 (Preparation of 5-methoxy-N-((R)-1-phenylethyl)-1,2,3,4-tetrahydronaphthalene-2-amine hydrochloride)
[0056] Dissolve MPTN-0 (0.948mol, 167.0g) in 1.4kg of absolute ethanol, and add R-α-methylbenzylamine (1.137mol, 137.8g) dropwise at room temperature (25°C). After the addition is complete, Raise the temperature to 30~35°C and stir for 5h; after the stirring is finished, cool down to 5~10°C, add sodium borohydride (0.948mol, 35.9g) in batches, control the temperature of the system at 10~15°C, and stir for 6h; the stirring is over Finally, keep the temperature of the system at 10-15°C, add 200g of concentrated hydrochloric acid dropwise to adjust the pH of the system to <3, stir for 2 hours, a large amount of solids precipitate, filter, and dry to constant weight to obtain 270.0g of off-white solid MPTN-1, with a yield of 89.6 %, HPLC analysis purity is 98.2%.
Embodiment 2
[0057] Example 2 (preparation of 5-methoxy-1,2,3,4-tetrahydronaphthalene-2-amine)
[0058] Add MPTN-1 (270.0g, 0.85mol) into a 5L hydrogenation kettle, add 2kg of methanol, 25g of 10% palladium carbon wet product, control the hydrogen pressure at 2~5atm, stir at 60°C for 5h, and the debenzylation is completed. Add sodium hydroxide powder equivalent to MPTN-1 into the reaction kettle, and stir for 3h. Then the reaction solution was filtered, and the solvent was removed from the filtrate under reduced pressure to obtain 100.0 g of brown oil MPTN-2 with a yield of 66.4% and a purity of 97.6% by HPLC analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com