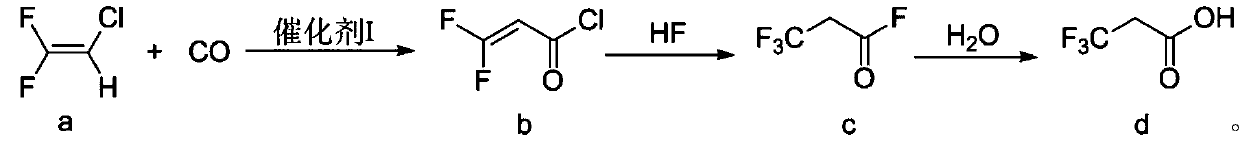
Preparation method of 3, 3, 3-trifluoropropionic acid
A technology of trifluoropropionic acid and trifluoropropionyl, which is applied in the field of preparation of 3,3,3-trifluoropropionic acid, can solve the problems of expensive raw materials and reagents, low yield and the like, and achieves improved conversion rate and selectivity, The effect of high conversion and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] This embodiment includes the following steps:
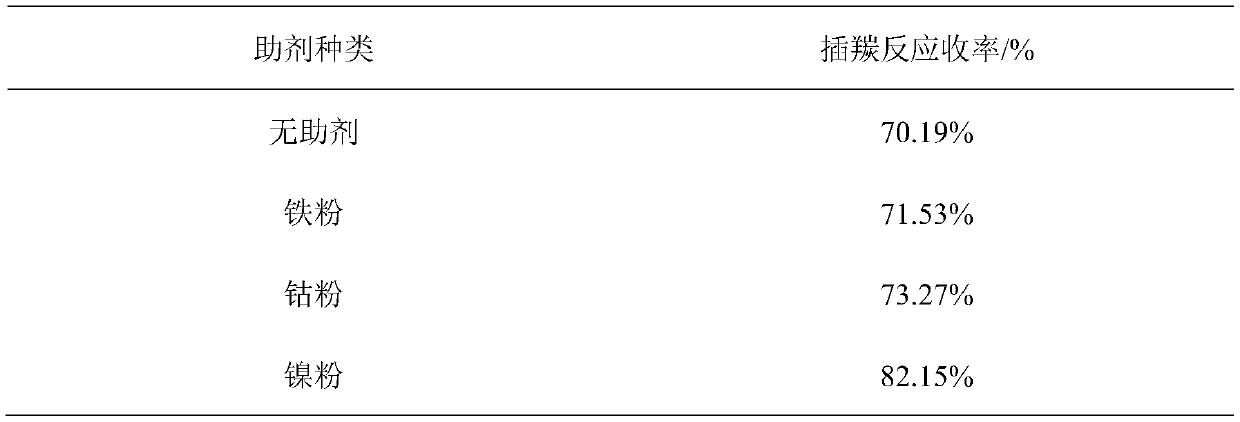
[0037] (1) Add 4.5g (25.4mmol) palladium chloride, 1.5g (25.6mmol) nickel powder and 13.3g (50.8mmol) triphenylphosphine in 2L stainless steel reaction kettle, vacuumize, draw in 1000mL anhydrous tetrahydrofuran, Feed 250g (2.54mol) of 2-chloro-1-1-difluoroethylene under stirring, raise the temperature to 80°C, feed carbon monoxide to a pressure of 0.8 MPa, keep warm until the pressure no longer drops, and then continue to feed carbon monoxide to a pressure of 0.8 MPa , pass through three times, pass through time 5 hours, stop reaction. Cool down to room temperature, filter and recover divalent palladium and nickel powder, quickly distill the filtrate under normal pressure to obtain a mixed solution of organic solvent and product, then rectify and recover the organic solvent, collect the fraction at 70-72°C, which is 3,3-difluoro- 2-acryloyl chloride weighs 263.8g, and the yield is 82.15%.
[0038] (2) Vacuumize the 2L M...
Embodiment 2
[0041] This embodiment includes the following steps:
[0042](1): Add 6.3g of palladium acetate (28.1mmol), 1.7g of nickel powder (29.0mmol) and 14.6g of triphenylphosphine (55.6mmol) into a 2L stainless steel reaction kettle, vacuumize, and suck in 1000mL of anhydrous tetrahydrofuran, Feed 275g (2.79mol) of 2-chloro-1-1-difluoroethylene under stirring, raise the temperature to 90°C, feed carbon monoxide to a pressure of 0.8 MPa, keep warm until the pressure no longer drops, and then continue to feed carbon monoxide to a pressure of 0.8 MPa , pass through three times, pass through time 5 hours, stop reaction. Cool down to room temperature, filter and recover divalent palladium and nickel powder, quickly distill the filtrate under normal pressure to obtain a mixed solution of organic solvent and product, then rectify and recover the organic solvent, collect fractions at 70-72°C to obtain 3,3-difluoro-2- Acryloyl chloride weighs 281.9g, and the yield is 79.8%.
[0043] (2): Va...
Embodiment 3
[0046] This embodiment includes the following steps:
[0047] (1) Add 2.5g (14.1mmol) palladium chloride, 1.7g (29.0mmol) nickel powder and 15.3g (58.3mmol) triphenylphosphine in 2L stainless steel reactor, vacuumize, suck in 1240mL anhydrous tetrahydrofuran, Feed 275g (2.79mol) of 2-chloro-1-1-difluoroethylene under stirring, raise the temperature to 80°C, feed carbon monoxide to a pressure of 0.8 MPa, keep warm until the pressure no longer drops, then continue to feed carbon monoxide to a pressure of 0.8 MPa , pass through three times, pass through time 6 hours, stop reaction. Cool down to room temperature, filter and recover divalent palladium and nickel powder, quickly distill the filtrate under normal pressure to obtain a mixed solution of organic solvent and product, then rectify and recover the organic solvent, collect the fraction at 70-72°C, which is 3,3-difluoro 2 - acryloyl chloride, heavy 278g, yield 78.7%.
[0048] (2) Vacuumize the 2L Monel reactor, pump in 278...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com