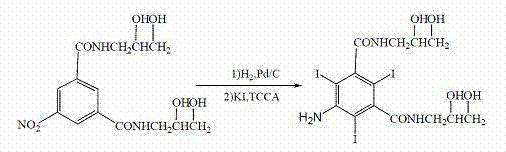
Improved preparation process of 5- amino-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzene dicarboxamide
A technology of phthalamide and dihydroxypropyl, which is applied to the preparation of carboxylic acid amides, the preparation of organic compounds, chemical instruments and methods, etc., which can solve the problem of low yield of the preparation method, difficult control of the production process, and danger to operators and other problems to achieve the effect of good reaction selectivity, high production cost and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The total amount of 5-amino-N, N'-bis(2,3-dihydroxypropyl)-1,3-benzenedicarboxamide aqueous solution obtained in the reaction was added to 340 grams of potassium iodide, 50 grams of sodium hydroxide, and 120 grams of trichloro Isocyanuric acid (TCCA), stirred and reacted at 0°C for 10 minutes, adjusted the pH of the solution to 5-7 with 25% sodium hydroxide, precipitated a solid, filtered to obtain 249 g of the product, melting point 176-178°C, yield 63.1%.
Embodiment 2
[0024] The total amount of 5-amino-N, N'-bis(2,3-dihydroxypropyl)-1,3-benzenedicarboxamide aqueous solution obtained in the reaction was added to 170 grams of potassium iodide, 50 grams of sodium hydroxide, and 40 grams of trichloro Isocyanuric acid (TCCA), stirred and reacted at 25°C for 100 minutes, adjusted the pH of the solution to 5-7 with 25% sodium hydroxide, precipitated a solid, and filtered to obtain 302 g of the product, with a melting point of 176-178°C and a yield of 76.5%.
Embodiment 3
[0026] The total amount of 5-amino-N, N'-bis(2,3-dihydroxypropyl)-1,3-benzenedicarboxamide aqueous solution obtained in the reaction was added to 250 grams of potassium iodide, 50 grams of sodium hydroxide, and 100 grams of trichloro Isocyanuric acid (TCCA), stirred and reacted at 0°C for 60 minutes, adjusted the pH of the solution to 5-7 with 25% sodium hydroxide, precipitated a solid, and filtered to obtain 319 g of the product, with a melting point of 176-178°C and a yield of 80.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com