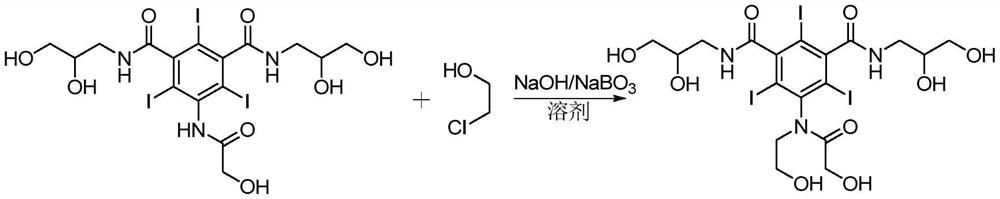
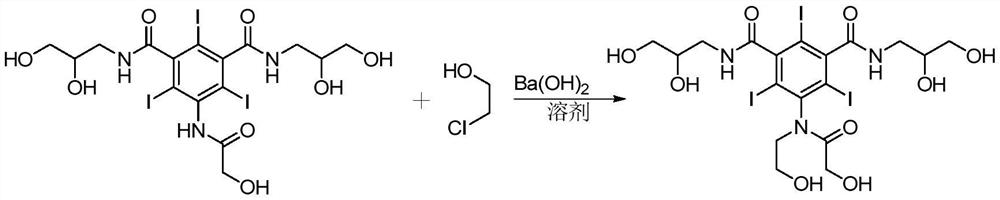
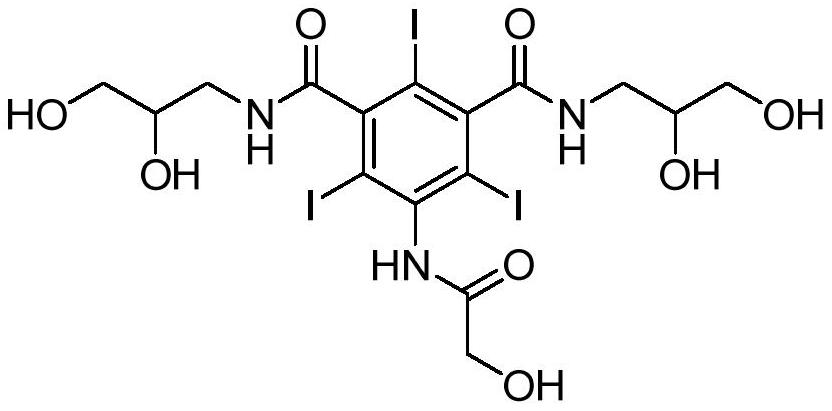
A kind of method for preparing ioversol
A technology of ioversol and iodoethanol, which is applied in the field of preparation of ioversol, can solve the problems of increased production cost, large amount of alkali used, difficult recovery and mechanical application, etc., and achieves the effects of less impurities, mild reaction conditions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Preparation of ioversol
[0029] In a 1000 mL reaction kettle, add 200 mL of methanol, 10.6 g (0.075 mol) of disodium hydrogen phosphate, and 76.3 g (0.1 mol) of compound I, start stirring, heat up to 30 °C, and after stirring evenly, slowly add chloroethanol dropwise Methanol solution (8.85g (0.11mol) of chloroethanol and 100ml of methanol), and 16.5g (0.035mol) of 30wt% disodium hydrogen phosphate solution was added dropwise at the same time, the pH of the reaction system was controlled at 12-13, and the dropwise addition was completed in 6h. Incubate the reaction for 24h. After cooling to room temperature, neutralize with dilute hydrochloric acid, filter, pass the filtrate through anion and cation exchange resin (special resin factory of Xi'an Lanxiao Technology Co., Ltd., cation exchange resin D101, anion exchange resin D201) for desalting, and the filtrate is concentrated and recrystallized with methanol 3 76.7 g of ioversol was obtained, HPLC≥99.5%, an...
Embodiment 2
[0031] Example 2: Preparation of ioversol
[0032] In a 1000 mL reaction kettle, add 200 mL of methanol, 13.1 g (0.075 mol) of dipotassium hydrogen phosphate, 76.3 g (0.1 mol) of compound I, turn on stirring, heat up to 35°C, and after stirring evenly, slowly add chloroethanol dropwise Methanol solution (8.85g (0.11mol) of chloroethanol and 100ml of methanol) was added dropwise with 20.9g (0.036mol) of 30wt% dipotassium hydrogen phosphate solution, the pH of the reaction system was controlled at 12-13, and the dropwise addition was completed in 6h. Incubate the reaction for 28h. After being cooled to room temperature, neutralized with dilute hydrochloric acid, filtered, the filtrate was desalted through anion and cation exchange resin (same as in Example 1), the filtrate was concentrated and recrystallized with methanol 3 times to obtain ioversol 78.6g, HPLC≥99.5%, yield 97.4%.
Embodiment 3
[0033] Example 3: Preparation of ioversol
[0034] In a 1000mL reaction kettle, add 200mL of water, 11.8g (0.068mol) of dipotassium hydrogen phosphate, 76.3g (0.1mol) of compound I, start stirring, heat up to 25°C, and after stirring evenly, slowly add chloroethanol dropwise Aqueous solution (9.66g (0.12mol) of chloroethanol and 100ml of water), 34.2g (0.059mol) of 30wt% dipotassium hydrogen phosphate solution was added dropwise at the same time, the pH of the reaction system was controlled at 12-13, and the dropwise addition was completed in 6h. Incubate the reaction for 30h. After being cooled to room temperature, neutralized with dilute hydrochloric acid, filtered, the filtrate was desalted through anion and cation exchange resin (same as Example 1), the filtrate was concentrated and recrystallized with water 3 times to obtain ioversol 79g, HPLC≥99.5%, yield 98% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com