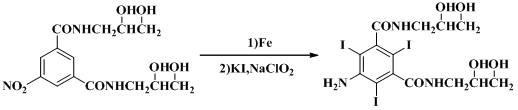
Method for preparing 5-amino-N,N'-di(2,3-dihydroxypropyl)-2,4,6-triiodoisophthal amide
A technology of triiodoisophthalamide and dihydroxypropyl, which is used in the preparation of 5-amino-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodoisophthalamide In the field of preparation, it can solve problems such as high production costs, dangerous operators, and complex preparation processes, and achieve the effects of reducing environmental impact, production costs, and equipment corrosion.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 16.5 grams of potassium iodide and 1M hydrochloric acid to the 5-amino-N, N'-bis(2,3-dihydroxypropyl) isophthalamide solution obtained in the reduction reaction to adjust the pH to 1-2, and add 4.4 grams of sodium chlorite , stirred and reacted at 0°C for 1 hour, adjusted the pH of the solution to neutral with 1M NaOH, left the filtrate at room temperature to precipitate a solid, filtered, and dried at 70°C to obtain 18.2 g, with a yield of 51.7%.
Embodiment 2
[0021] Add 33.1 grams of potassium iodide and 1M hydrochloric acid to the 5-amino-N, N'-bis(2,3-dihydroxypropyl)isophthalamide solution obtained in the reduction reaction to adjust the pH to 1-2, and add 17.8 grams of sodium chlorite , stirred and reacted at 50°C for 8 hours, adjusted the pH of the solution to neutral with 1M NaOH, left the filtrate at room temperature to precipitate solids, filtered, and dried at 70°C to obtain 21.2 g, with a yield of 60.3%.
Embodiment 3
[0023] Add 21.4
[0024] Adjust the pH to 1-2 with 1 g of potassium iodide and 1M hydrochloric acid, add 5.3 g of sodium chlorite, stir and react at 15°C for 3 hours, adjust the pH of the solution to neutral with 1M NaOH, leave the filtrate at room temperature to precipitate solids, filter, and dry at 70°C , to obtain 25.5 grams, yield 72.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com