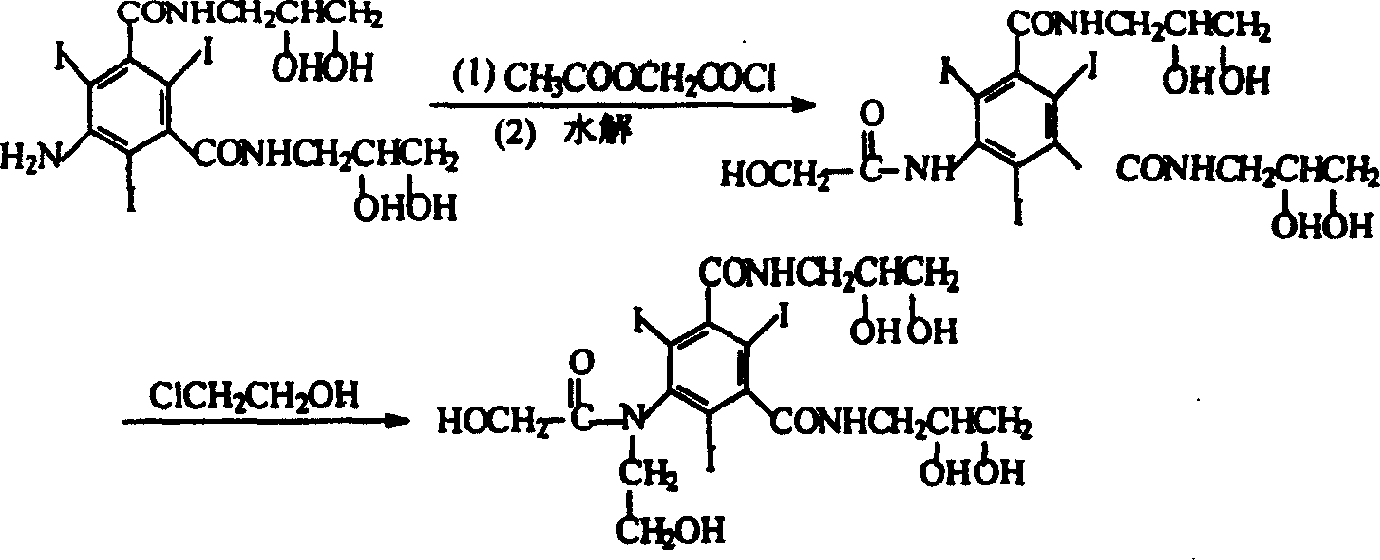
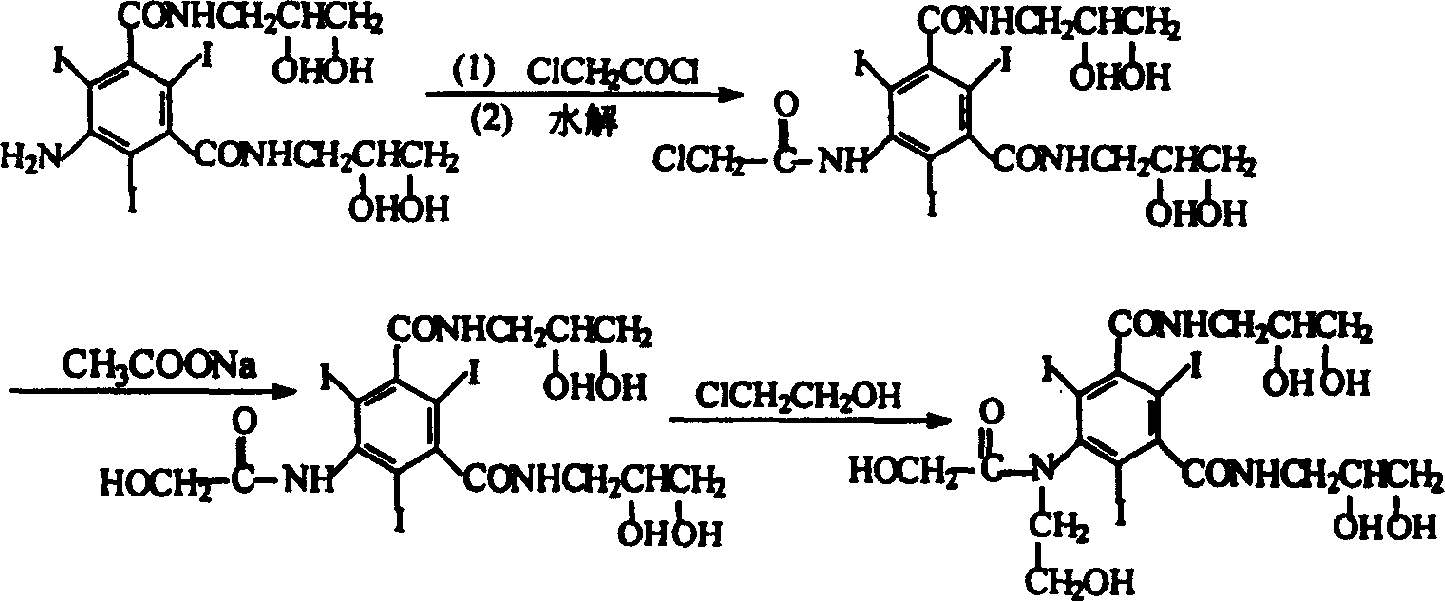
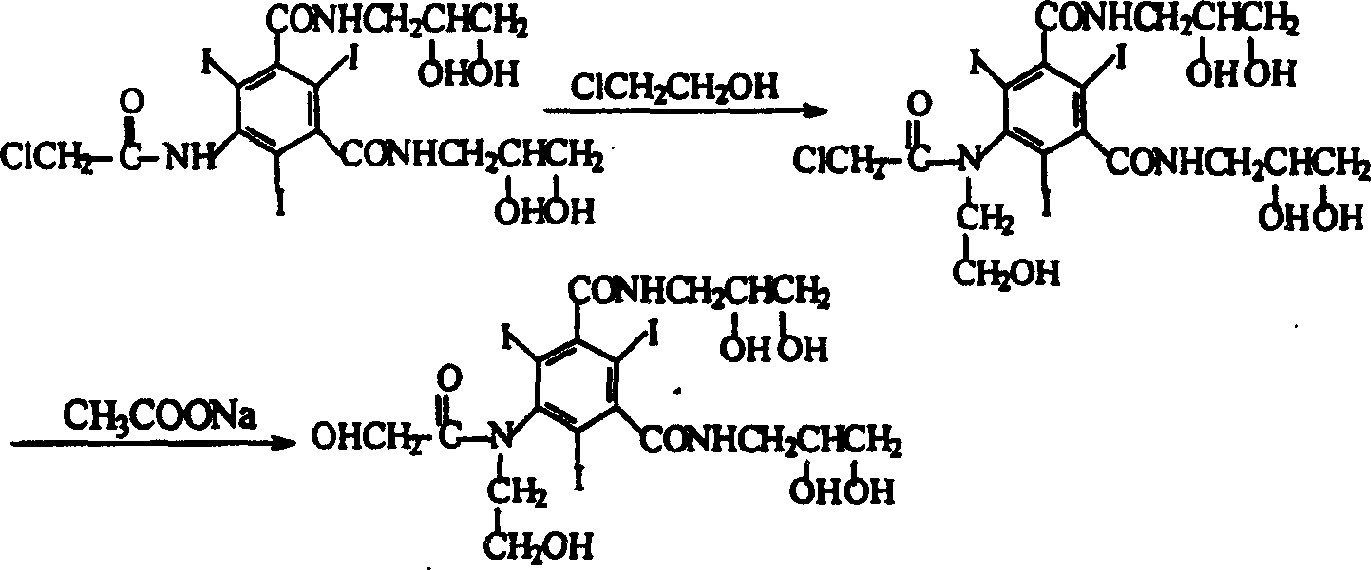
Preparation method of ioversol
A technology of ioversol and chloroethanol, applied in the field of preparation of western medicine compounds, can solve the problems of difficult control of reaction conditions, long reaction time and the like, and achieves the effects of low cost, simple operation and low equipment investment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] In a three-necked flask equipped with a stirrer and a reflux condenser, add 50 g (0.064 mol) of 5-chloroacetamido-N, N'-bis(2,3-dihydroxypropyl)-2,4,6- Triiodo-1,3-benzenedicarboxamide, 9g (0.16mol) potassium hydroxide, 250ml water, after all dissolved, add 14g (0.174mol) chloroethanol, stir at 50°C for 15 hours, add 5mol / L hydrochloric acid solution Adjust the pH value of the reaction solution to about 6.0 without purification, directly add 26 g (0.317 mol) of anhydrous sodium acetate to the reaction solution, and add concentrated hydrochloric acid dropwise under stirring to adjust the pH value to 6.5. Heat to reflux, and reflux for 20 hours, during which 5mol / L sodium hydroxide solution is added appropriately to maintain the pH value of the reaction solution at 5.5-6.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com