Completely-biodegradable support capable of developing and preparation method thereof
A complete, stent rod technology, applied in the field of medical devices, can solve the problems of easy falling off and disappearance of the coating, weak bonding strength of the stent body, etc., achieving good biocompatibility and avoiding adverse reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
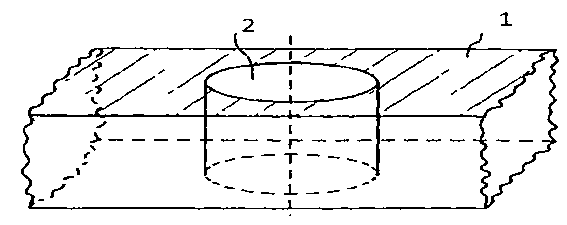
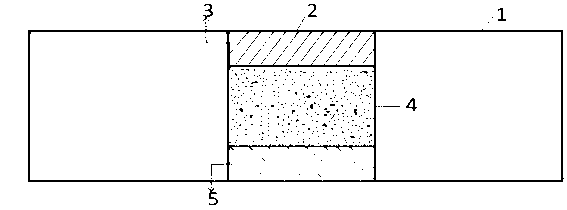
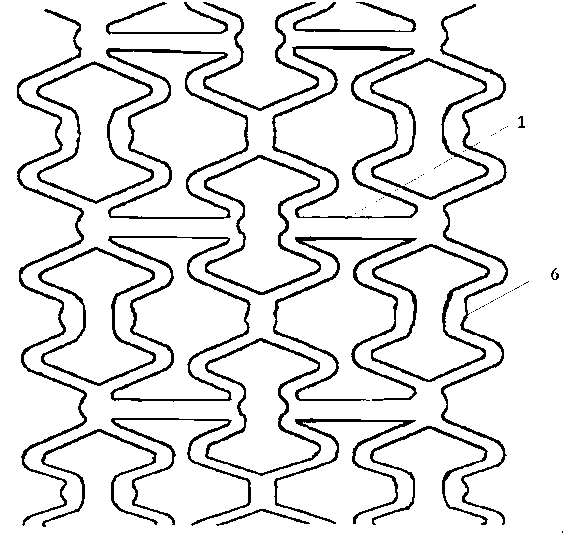
[0050] The material of the stent body in the embodiment of the present invention is biodegradable polymer material PCL (polycaprolactone), and the overall schematic diagram of the stent is as follows Figure 6 shown. A hexagonal hole is centrally arranged in the length and width directions of all connecting straight rods 1 of the bracket, wherein each connecting straight rod has a hole, such as figure 1 and Figure 7 shown. The diagonal of the hexagonal hole is about 80 μm, which is about 2 / 5 of the width of the connecting straight rod. Among them, the degradable polymer used in the protective layer is PLLA (L-polylactic acid), with a weight average molecular weight of Mw=230,000 to 250,000, which can be dissolved in acetone to form a viscous solution with a mass fraction of 70% to 95%. The X-ray opaque contrast agent used in the developing layer is the purified crystal of diatrizoic acid, which is dissolved in ethanol to form a solution with a mass fraction of 60% to 95%. ...
Embodiment 2
[0053] The material of the stent body in the embodiment of the present invention is a copolymer of biodegradable polymer materials PLLA (poly-L-lactic acid) and PCL (polycaprolactone). The overall schematic diagram of the stent is as follows Figure 6 shown. A round hole is centrally arranged in the width direction of all connecting straight rods 1 of the bracket, wherein each connecting straight rod has 4 holes, and straight lines are evenly distributed on the connecting long straight rods, such as figure 2 shown. The pore diameter is about 90 μm, which is about 1 / 2 of the width of the stent rod. Among them, the degradable polymer PCL (polycaprolactone) used in the inner protective layer 5, the outer protective layer 3 is PLGA (polylactic acid-glycolic acid), the weight average molecular weight Mw = 170,000 to 190,000, and is soluble in acetone to form a mass Fraction 70%~95% viscous solution. The X-ray opaque contrast agent used in the developing layer is the purified cr...
Embodiment 3
[0056] The material of the stent body in the embodiment of the present invention is biodegradable polymer material PLLA (poly-L-lactic acid), and the overall schematic diagram of the stent is as follows Figure 6 shown. Round holes are provided on all connecting rods of the bracket, and each connecting rod has 3 holes, and a triangular arrangement is adopted on the connecting rods, such as image 3 shown. The diameter of the hole is about 50 μm, which is about 1 / 4 of the width of the connecting straight rod. Among them, the degradable polymer used in the protective layer is PLGA (polylactic acid-glycolic acid), with a weight average molecular weight of Mw=100,000 to 120,000, which can be dissolved in acetone to form a viscous solution with a mass fraction of 70% to 95%. The X-ray opaque contrast agent used in the developing layer is the purified crystal of iohexol, which is dissolved in ethanol to form a solution with a mass fraction of 60% to 95%.
[0057]First, a stainles...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com