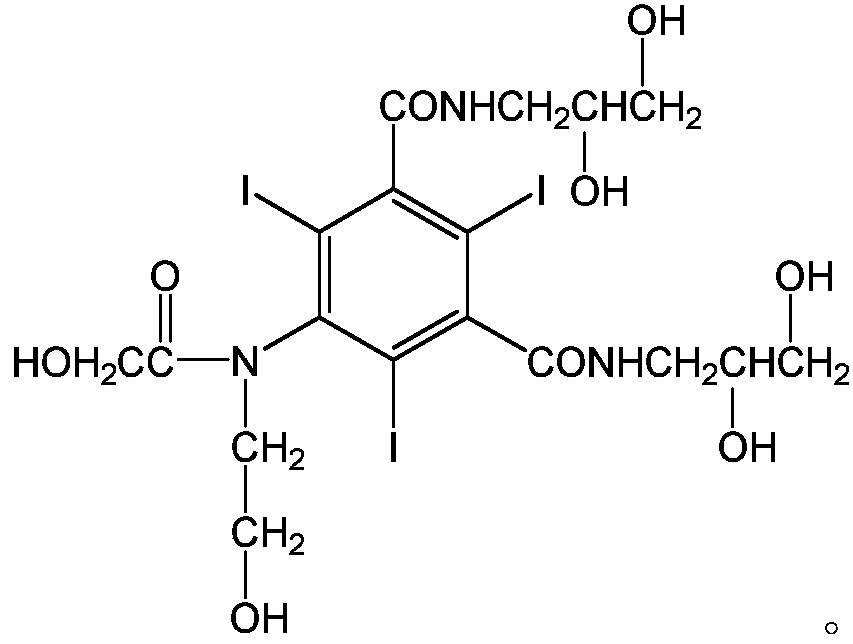
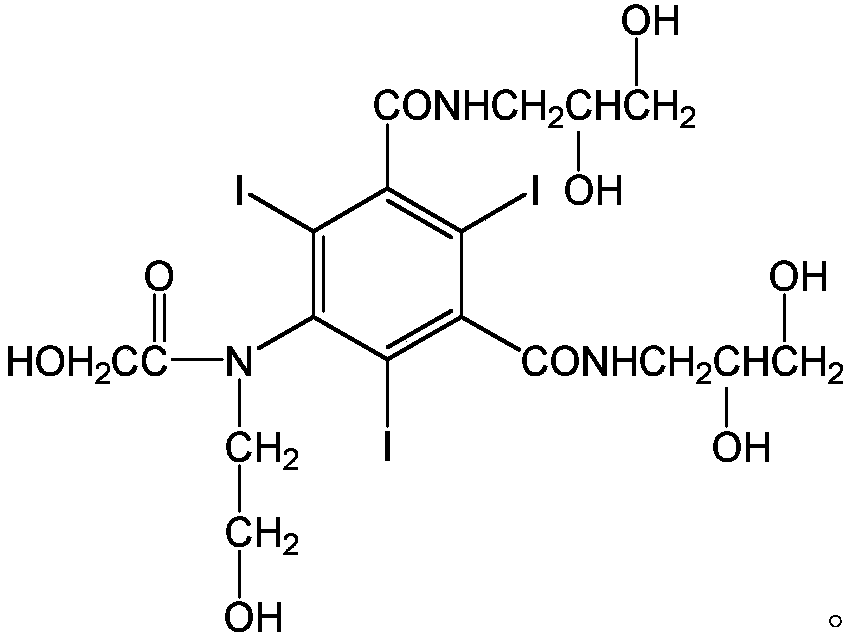
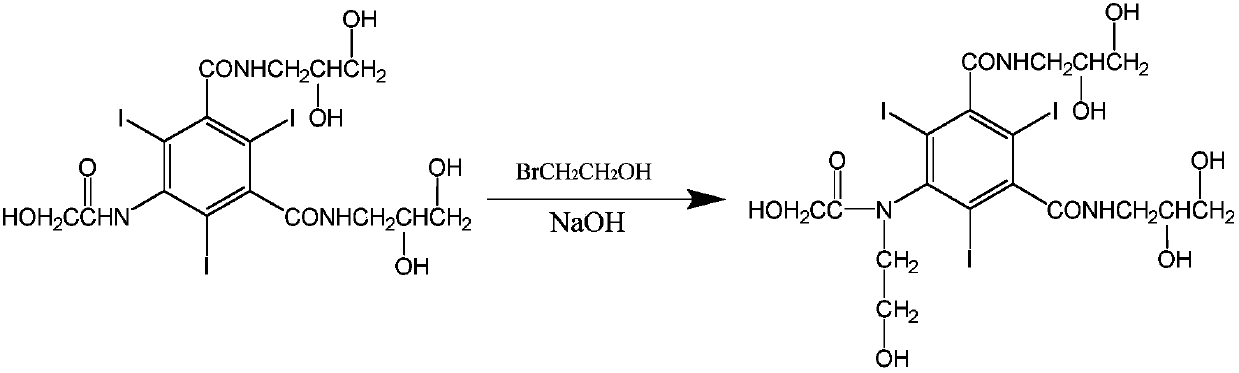
Ioversol and synthesis method thereof
A synthetic method, the technology of ioversol, which is applied in the field of organic compound preparation, can solve the problems of reducing the utilization rate of raw materials, prone to hydrolysis, storage difficulties, etc., and achieve the effect of improving the utilization rate of raw materials, reducing the amount of solvent used, and reducing potential safety hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 10g of 5-chloroacetamido-N,N'-bis-(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide to 250ML four-port burnt product, 10g ethylene glycol, 10g absolute ethanol, 0.5g sodium hydroxide, heat to dissolve, then add 3.24g bromoethanol, react at 45°C for 24 hours, after the reaction, evaporate the solvent under reduced pressure, then dissolve with methanol, filter , the filtrate was evaporated to remove methanol under reduced pressure, 10.3 g of vacuum-dried solids were dissolved in 10 g of water, passed through a macroporous resin column, and the filtrate was evaporated to dryness under reduced pressure to obtain 9.3 g of solids, which were detected by HPLC at 98.4%, and then water (3 mL) and n-butyl Alcohol (10 mL) was recrystallized to obtain 7.9 g of ioversol with a purity of 99.3% by HPLC.
Embodiment 2
[0034] Add 100kg of 5-chloroacetamido-N,N'-bis-(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide, 300g of ethylene glycol, 100kg of absolute ethanol, and 5kg of sodium hydroxide were heated to dissolve, then 32.4kg of bromoethanol was added, and reacted at 45°C for 24 hours. After the reaction was completed, the solvent was evaporated under reduced pressure, then dissolved in methanol, and filtered. The filtrate was decompressed to evaporate methanol, and 105 kg of vacuum-dried solids were dissolved in 100 kg of water, passed through a macroporous resin column system, and the liquid was spray-dried to obtain 94.7 kg of solids, which were detected by HPLC at 98.47%, and then water (30 L) and n-butyl Alcohol (80 L) was recrystallized to obtain 83.1 kg ioversol, the purity of which was 99.35% by HPLC.
Embodiment 3
[0036] Add 10g of 5-chloroacetamido-N,N'-bis-(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide to 250ML four-port burnt product, 50g ethylene glycol, 10g absolute ethanol, 0.5g sodium hydroxide, heat to dissolve, then add 3.24g bromoethanol, react at 45°C for 24 hours, after the reaction is completed, evaporate the solvent under reduced pressure, then dissolve with methanol, filter , the filtrate was evaporated to remove methanol under reduced pressure, and 8.9 g of vacuum-dried solids were dissolved in 10 g of water, passed through a macroporous resin column, and the filtrate was evaporated to dryness under reduced pressure to obtain 7.3 g of solids, which were detected by HPLC at 97.98%, and then water (3 mL) and n-butyl Alcohol (10 mL) was recrystallized to obtain 6.8 g of ioversol with a purity of 99.1% by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com