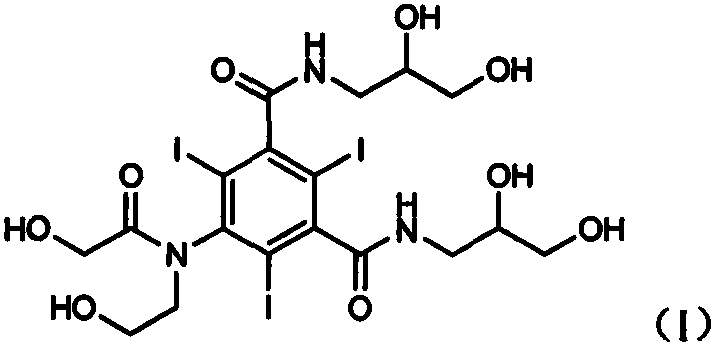
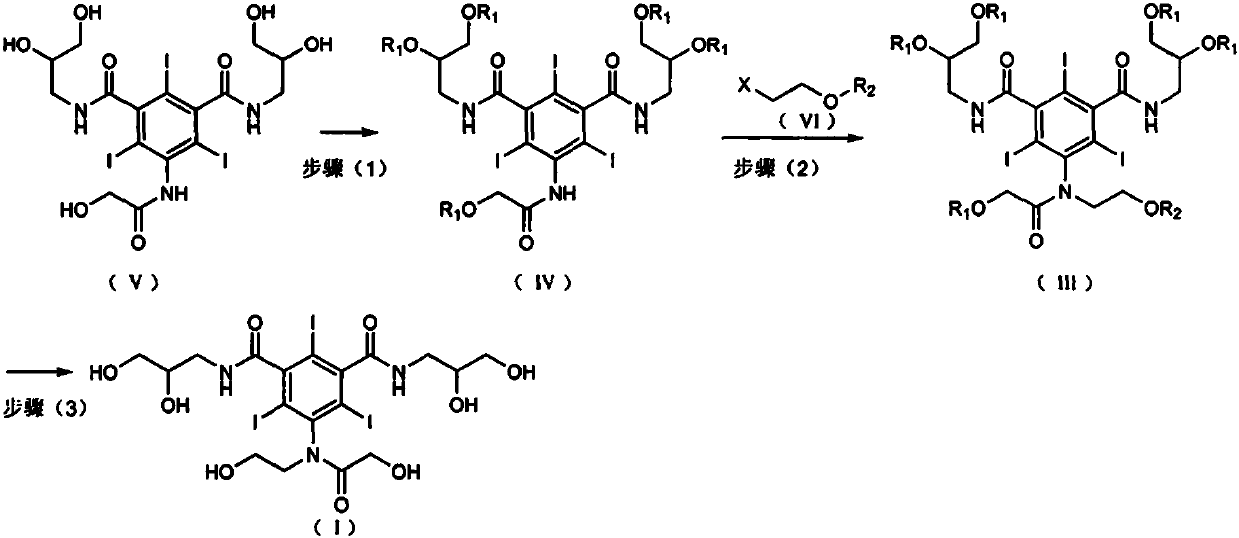
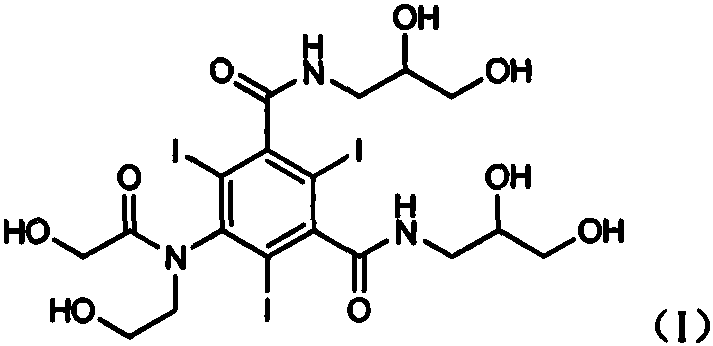
Preparation method of ioversol
A technology of ioversol and acid-binding agent, which is applied in the field of preparation of ioversol, to achieve the effects of reducing the number of refining times, avoiding rearrangement of impurities, and reducing the content of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1 (preparation of formula (IV))
[0039]Add formula (V) 5-hydroxyacetamido-N, N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedimethanol in a 5L reaction flask Stir and dissolve amide 1kg and 2L dimethyl sulfoxide, add 1.5kg of anhydrous potassium carbonate and cool down to 20°C, add 1.1kg of tert-butyldimethylsilyl chloride dropwise, after the addition is complete, control the temperature at 30-35°C and stir for 10 hours . used directly in the next reaction.
Embodiment 2
[0040] Embodiment 2 (preparation of formula (IV))
[0041] 440 g of 2-bromoethyl acetate was added to the reaction solution obtained in Example 1, and the temperature was controlled at 30-35° C. and stirred for 16 hours. The insoluble matter was removed by filtration, and the filtrate was directly used in the next reaction.
Embodiment 3
[0042] Embodiment 3 (Ioversol crude product preparation)
[0043] Add the filtrate obtained in Example 2 to 1% sulfuric acid aqueous solution to adjust the pH value to 1-3, raise the temperature to 40-50° C. and stir for reaction, keep the pH value at 1-3 during the reaction process, and stir for 5 hours. After the reaction is complete, neutralize with 1% sodium hydroxide solution to pH 6-7. Concentrate under reduced pressure at 50-60°C until no obvious solvent is evaporated, add dichloromethane to the concentrated residue, stir and crystallize, filter, add methanol to the solid and stir well The insoluble matter was filtered off, and the filtrate was concentrated to dryness to obtain 861 g of crude ioversol, with a yield of 81.4%. (HPLC detection: purity 97.8%, impurity II content 0.39%)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com