A sulfate salt of an intestinal type 2b sodium phosphate cotransporter inhibitor and its crystalline form
A technology of crystallization and sulfuric acid, which is applied in the field of sulfate and its crystal form of intestinal 2B sodium phosphate cotransporter inhibitors, can solve problems such as easy caking, poor product stability, and difficult filtration, and achieve repeatable and reproducible production processes control, good crystal form stability, and stable production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
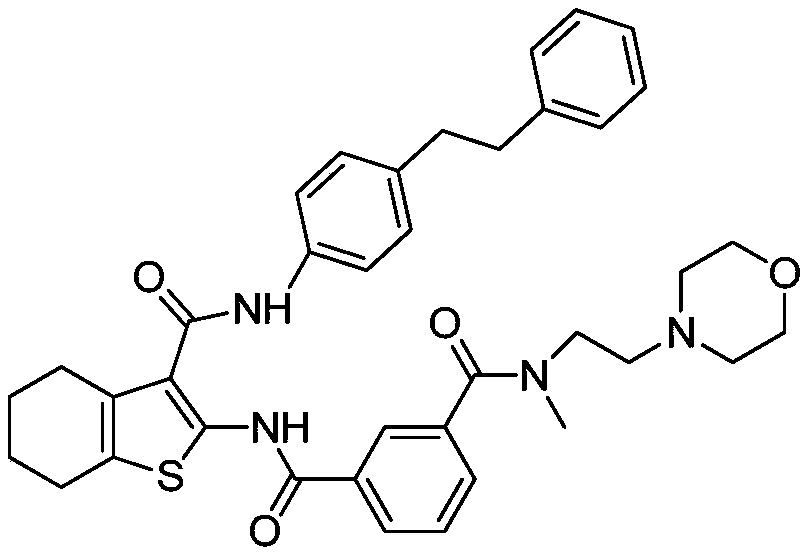
[0036] N 1 -Methyl-N 1 -(2-morpholine ethyl)-N 3-Synthesis of (3-(4-phenylethylphenylcarbamoyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)isophthalamide
[0037]
[0038] The first step: the synthesis of 2-cyano-N-(4-phenethylphenyl)acetamide
[0039] 4-Phenylethylaniline a (2.15g, 10.91mmol, prepared by the well-known method literature "Journal of Medicinal Chemistry, 56(5), 2139-2149; 2013") and 2-cyanoacetic acid (1.39g, 16.37mmol) was dissolved in 5mL N,N-dimethylformamide, cooled to 0°C, added 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (3.14g, 16.37 mmol), rose to room temperature, and stirred for 2h. Add 10mL of water, stir for 30min, solid precipitates, filter, and dry the filter cake to obtain the crude title product 2-cyano-N-(4-phenylethylphenyl)acetamide b (2.68g, white solid), the product is not After purification, proceed directly to the next reaction.
[0040] MS m / z(ESI):263.3[M-1]
[0041] The second step: the synthesis of 2-cyano-2-cyc...
Embodiment 2
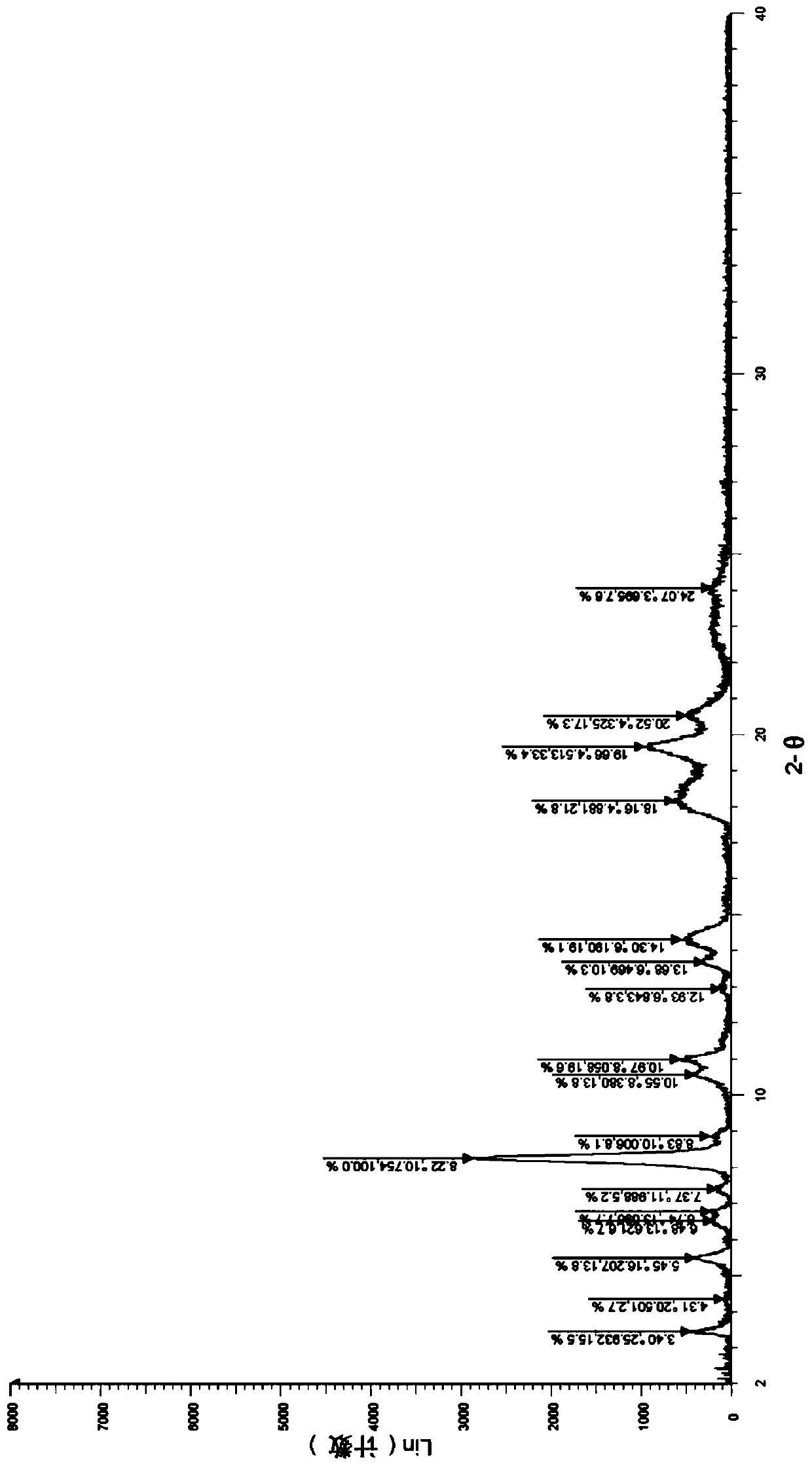
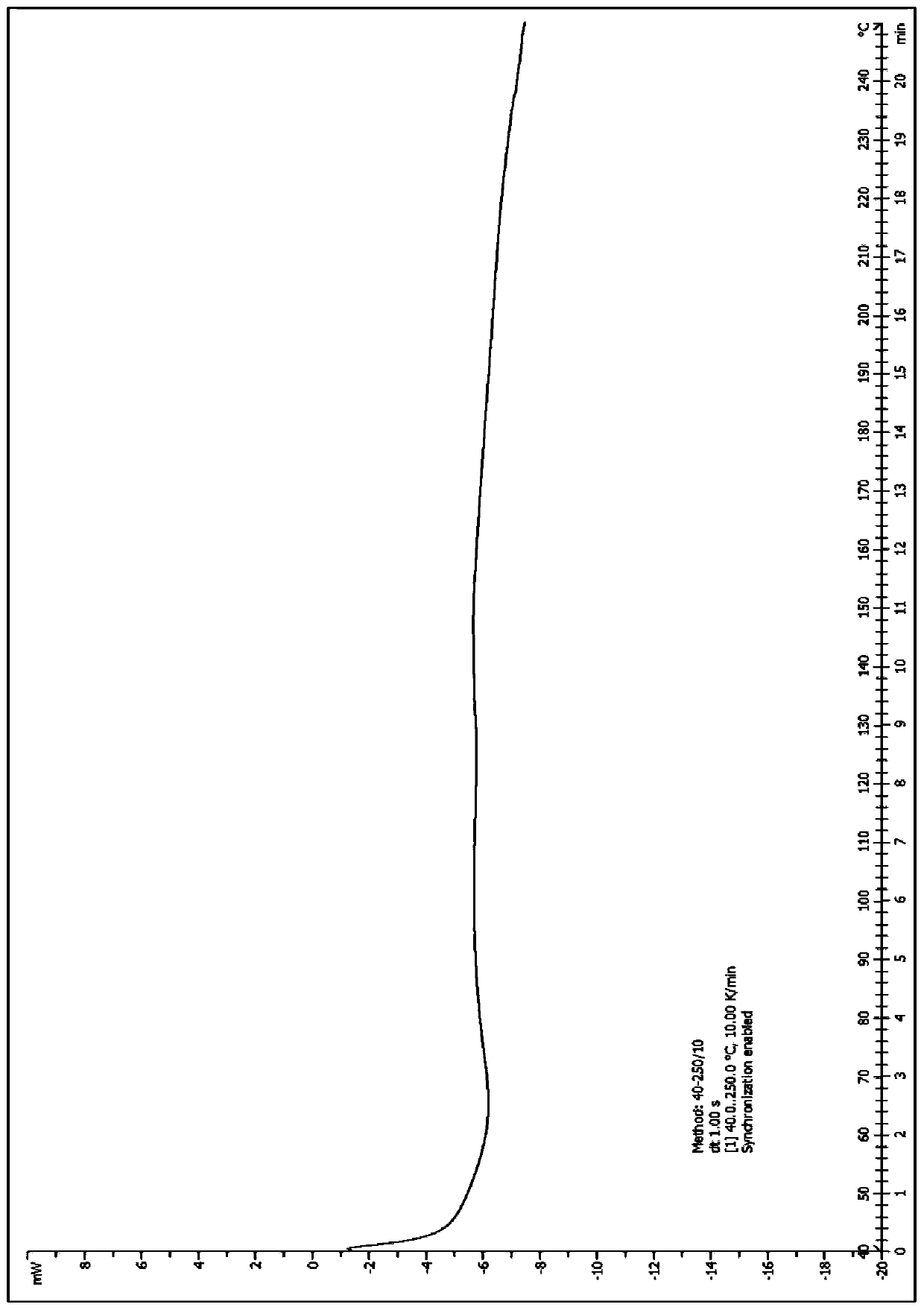
[0052] Take (500mg, 0.77mmol) N 1 -Methyl-N 1 -(2-morpholine ethyl)-N 3 -(3-(4-phenylethylphenylcarbamoyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)isophthalamide (prepared according to Example 1) In a 25mL single-necked bottle, add 5mL dimethyl sulfoxide, heat to dissolve at 40°C, then add 5mL water, then add sulfuric acid aqueous solution (27.5%, 356mg, 1.00mmol) dropwise, after dropping, react at 40°C for 1h, stop heating , stirred and crystallized. The next day, it was filtered with suction and dried to obtain 492 mg of solid, with a yield of 85.6%. For the X-ray diffraction of this crystalline sample see figure 1 where the There are characteristic peaks near (4.33). See the DSC spectrum figure 2 , DSC has no endothermic peak at <350°C, and this crystal form is defined as I crystal form.
Embodiment 3
[0054] Take (500mg, 0.77mmol) N 1 -Methyl-N 1 -(2-morpholine ethyl)-N 3 -(3-(4-phenylethylphenylcarbamoyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)isophthalamide (prepared according to Example 1) Add 5mL N,N-dimethylformamide to a 25mL single-necked bottle, heat to dissolve at 40°C, then add 5mL water, then add sulfuric acid aqueous solution (27.5%, 356mg, 1.00mmol) dropwise, and react at 40°C for 1h , stop heating, stir and crystallize. The next day, it was filtered with suction and dried to obtain 473 mg of solid, with a yield of 82.3%. The X-ray diffraction and DSC patterns of the crystalline sample are studied and compared, and it is determined that the product is the I crystal form.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com