Efficient contrast agent synthesizing method and application thereof
A synthesis method and contrast agent technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of reduced total reaction yield, long reaction route, utilization rate cannot reach 100%, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0195] The preparation of embodiment 1 intermediate mixture
[0196]
[0197] Method 1: Dissolve compound 2 in N,N-dimethylacetamide, under stirring, in order to make the reaction proceed more fully, you can add base to it to neutralize the hydrogen chloride generated in the reaction, and then add 3 -Amino-1,2-propanediol in N,N-dimethylacetamide solution, add and keep warm for 3 to 12 hours, then add dropwise 3-methylamino-1,2-propanediol in N,N-dimethylacetamide Base acetamide solution, after adding, keep warm for 3 to 12 hours, take a sample and measure the area percentage by HPLC. Material ratio and product ratio are shown in Table 1 (compound 2 equivalent is 1):
[0198] Table 1
[0199]
[0200]
[0201] As can be seen from the above table, when the reaction conditions are within the scope of the present invention, the content of one of the compounds of formula (I), formula (II) or formula (III) can be prepared to be greater than 50%, and, in the preferred Wi...
Embodiment 2
[0218] Example 2 intermediate preparation pilot test
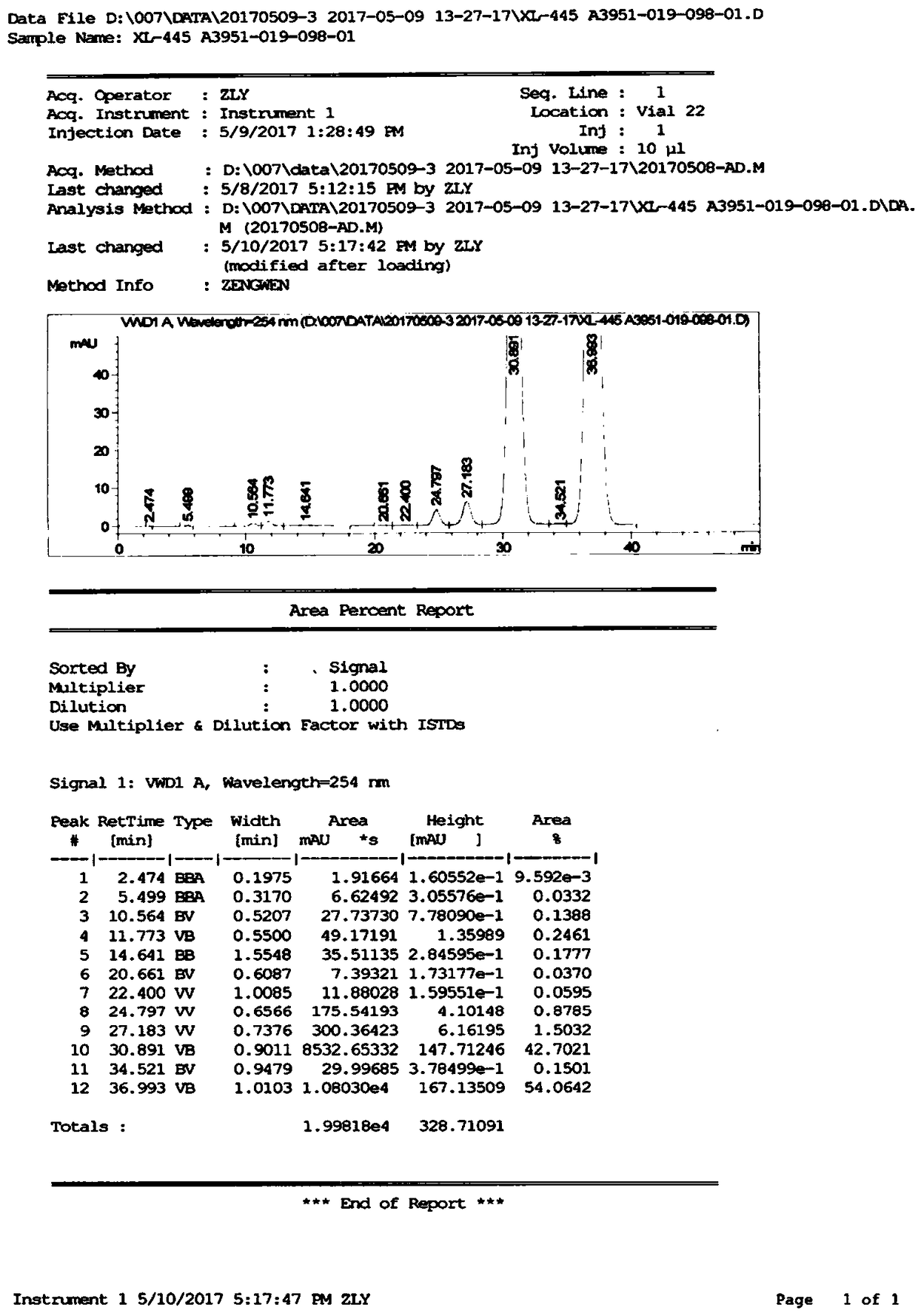
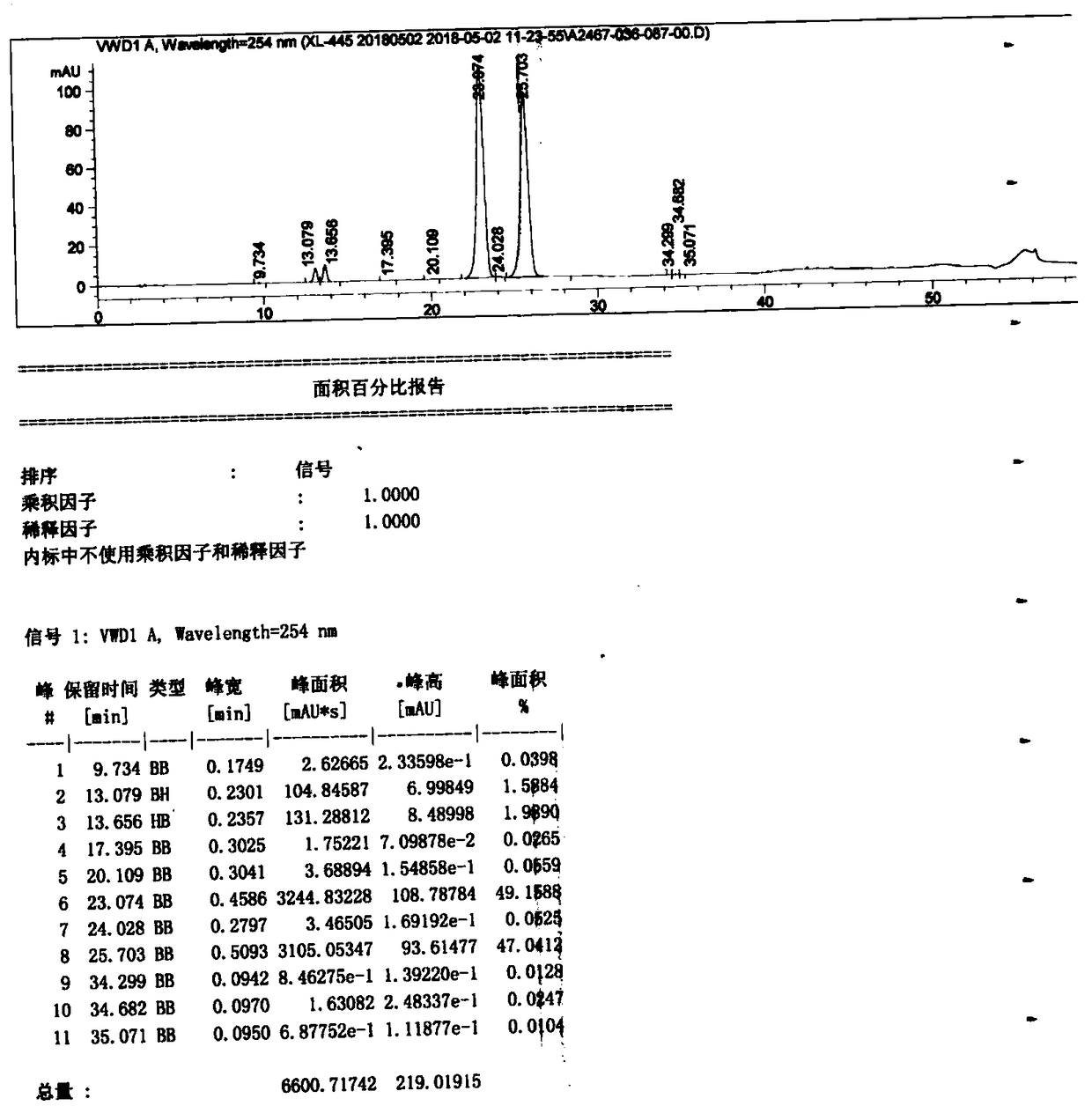
[0219] Compound 2 (5.96kg, 10.0mol) was dissolved in N,N-dimethylacetamide, under stirring, in order to make the reaction more fully, tri-n-propylamine (2.87kg, 20.0mol) was added thereto to neutralize Hydrogen chloride generated in the reaction, then added dropwise 3-amino-1,2-propanediol (911g, 10.0mol) in N,N-dimethylacetamide solution, reacted at 25-30°C for 12 hours, and then Add 3-methylamino-1,2-propanediol (2.1kg, 20.0mol) in N,N-dimethylacetamide solution dropwise. After the addition, keep the temperature for reaction for 12 hours. After the reaction is complete, concentrate under reduced pressure. The intermediate mixture obtained is water After dissolving, macroporous adsorption resin column chromatography, HPLC monitors the elution effect, respectively collects formula (I), formula (II), formula (III) compound fractions, and concentrates to obtain formula (I) white solid 768g (see figure 2 , Figure 5 ), the...
Embodiment 3
[0225] Embodiment 3 is synthesized contrast agent by intermediate
[0226] (1) Synthesis of iopromide
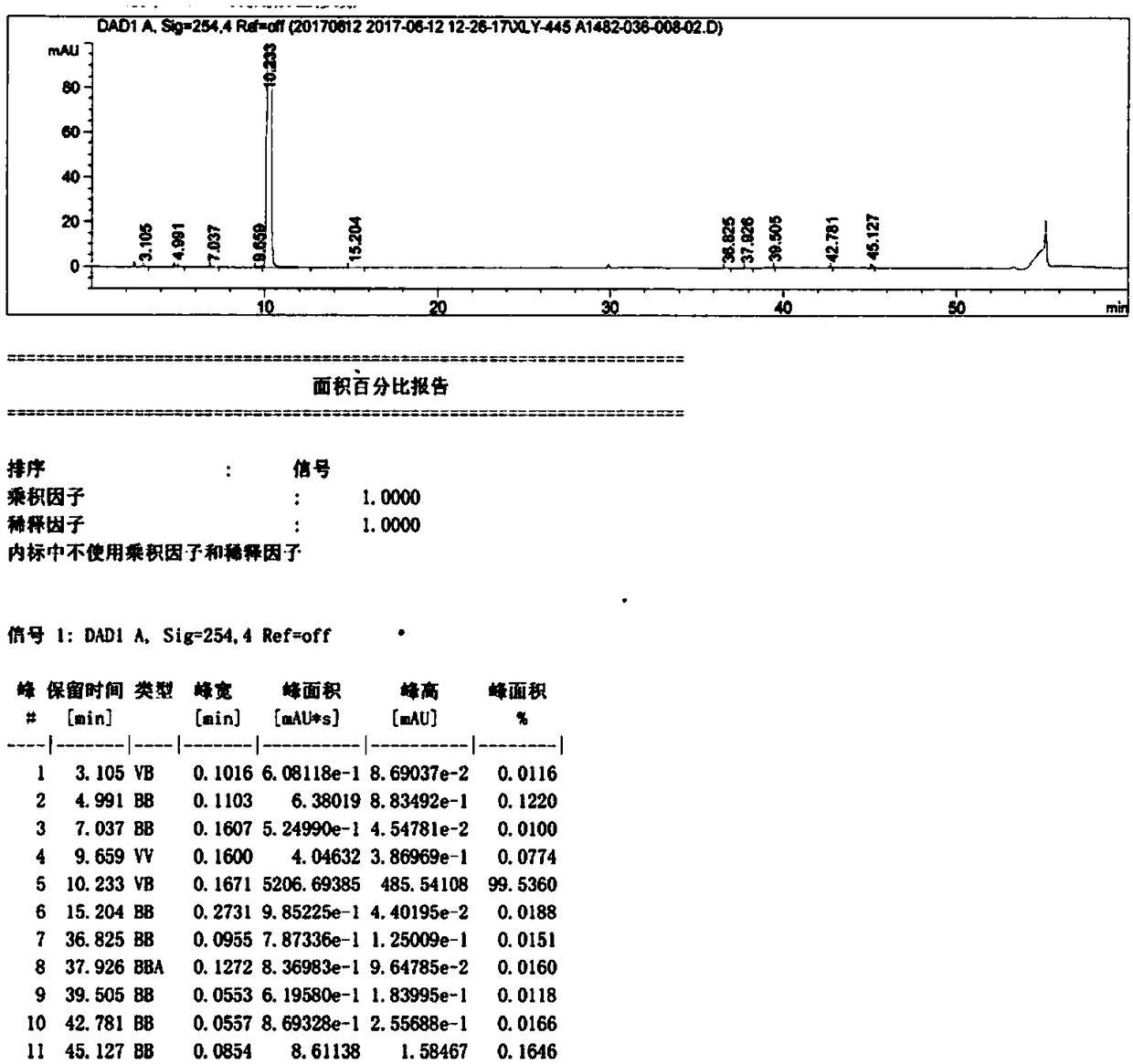
[0227] Take the compound of formula (II) (143.8g, 200mmol) into a 1L three-neck flask, add 290mL of ethyl acetate, 45mL of N,N-dimethylacetamide, acetic anhydride (91.8g, 0.9mol) and 4-dimethylaminopyridine (2.44g, 20mmol), magnetic stirring, overnight reaction at room temperature, TLC monitors the reaction until the raw material point and the intermediate state disappear, to obtain compound 50, [ 1 H NMR (400MH Z ,DMSO-d6):δ8.61~8.75(m,1H),5.56(s,2H),5.29(brs,1H),5.08(brs,1H),4.31~4.40(m,2H),4.16~4.22 (m,2H),3.69~3.77(m,1H),3.31~3.61(m,3H),2.80~2.83(m,3H),2.03~2.05(m,12H)];
[0228] Then, methoxyacetyl chloride (43.4 g, 400 mmol) was added to the reaction solution of compound 50, and the temperature was raised to reflux for 2-8 hours. TLC monitored the reaction until the raw material point disappeared. After the reaction, cool down to room temperature, add 200mL of puri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com