A kind of synthetic method and application of contrast agent intermediate
A technology for intermediates and contrast agents, applied in the field of synthesis of contrast agent intermediates, can solve the problems of unqualified color of finished products, long reaction routes, recovery and reuse of lost iodine, etc., and achieve simple routes, high separation and purification efficiency, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0195] Example 1 Preparation of Intermediate Mixture
[0196]
[0197] Method 1: The compound 2 is dissolved with N, N-dimethylacetamide, stirred, in order to make the reaction more fully proceed, the base can be added thereto, the hydrogen chloride formed in the neutralization reaction, then add 3 thereof. - N, N-dimethylacetamide solution of amino-1,2-propanediol, 2 ~ 12 hours of additional insulation reaction, then add 3-methyl amino-1,2-propanediol N, N-dimethyl The base of the aceralamide solution, the additional insulation reaction for 3 to 12 hours, and the amount of the Sampling HPLC was measured. The material ratio and product ratio are shown in Table 1 (2 equivalents of Compound 2):
[0198] Table 1
[0199]
[0200]
[0201] As can be seen from the above, when the reaction conditions are in the range of the present invention, one of the compounds of formula (I), the formula (II) or the formula (III), and in the preferred In the range, one of the compounds of formu...
Embodiment 2
[0218] Example 2 Intermediate system preparation
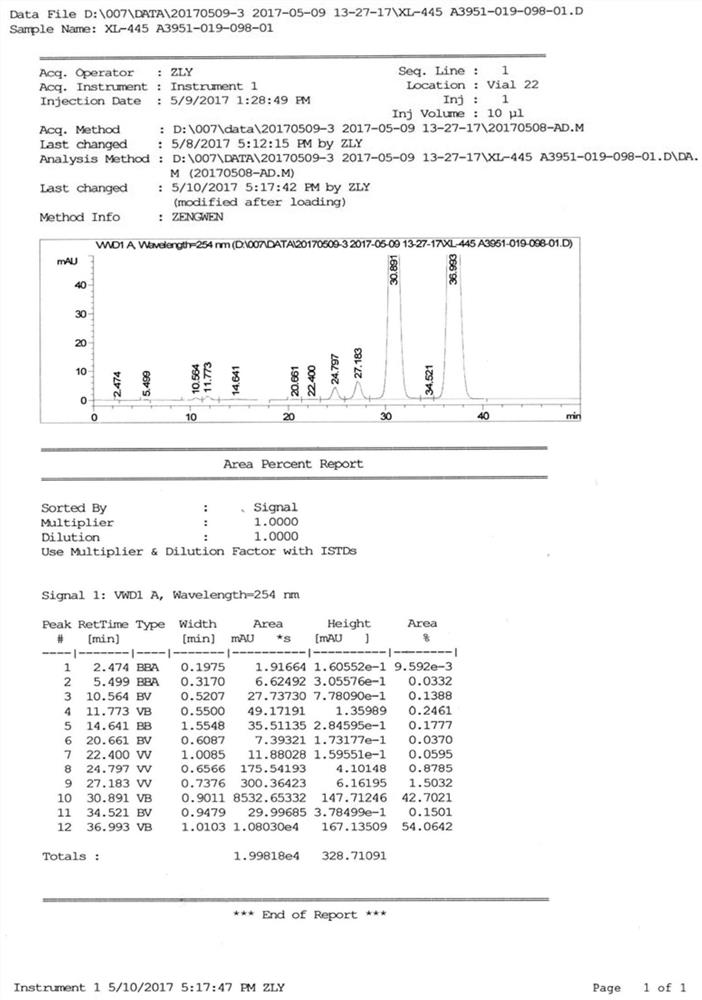
[0219] Compound 2 (5.96 kg, 10.0 mol) was dissolved with N, N-dimethylacetamide, stirring, in order to make the reaction performed more sufficiently, add triamine (2.87 kg, 20.0 mol), neutralized Hydrogen chloride produced in the reaction, then adding 3-amino-1,2-propylene glycol (911 g, 10.0 mol) of N, N-dimethylacetamide solution, adding 25 to 30 ° C for 12 hours, then then 3-methylene-1,2-propylene glycol (2.1 kg, 20.0 mol) of N, N-dimethylacetamide solution was added dropwise, and it was added for 12 hours, and the reaction was concentrated, and the resulting intermediate mixture was concentrated. After dissolving, the macroporous adsorption resin column chromatography, HPLC monitors elution effect, collected in formula (I), formula (II), formula (III), concentrated formula (I) white solid 768g (see figure 2 , Figure 5 The mass spectrometry and hydrogen spectrum data are as follows:
[0220] MS (ESI +): 705.8 [M + 1] + , 1 H N...
Embodiment 3
[0225] Example 3 Synthesis of Contrast
[0226] (1) Synthesis of iodobromide
[0227] The compound (II) compound (143.8 g, 200 mmol) was added to 1 L of three bottles, and 290 ml of ethyl acetate, 45 mln, N-dimethylacetamide, acetic anhydride (91.8 g, 0.9 mol) and 4-dimethylaminopyridine were added. (2.44 g, 20 mmol), magnetic stirring, room temperature reactions overnight, TLC monitoring reaction to the raw material point and the intermediate state disappeared, obtained compound 50, [ 1 H NMR (400MH Z DMSO-D6): Δ8.61 ~ 8.75 (m, 1H), 5.56 (S, 2H), 5.29 (BRS, 1H), 5.08 (BRS, 1H), 4.31 ~ 4.40 (m, 2H), 4.16 ~ 4.22 (M, 2H), 3.69 ~ 3.77 (m, 1H), 3.31 ~ 3.61 (m, 3H), 2.80 ~ 2.05 (m, 3H), 2.03 ~ 2.05 (m, 12H)];
[0228] The reaction solution to the compound 50 was added to methoxyacetyl chloride (43.4 g, 400 mmol), and the heated reflux reaction was 2-8h, and TLC was monitored to the reactive to the material point disappeared. After completion of the reaction, it was lowered to room temp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com