Method for synthesizing polysubstituted phosphono-1,3-butadiene compound
A compound, butadiene technology, applied in the field of organic synthesis and catalysis, can solve the problems of element waste, low yield, long reaction time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
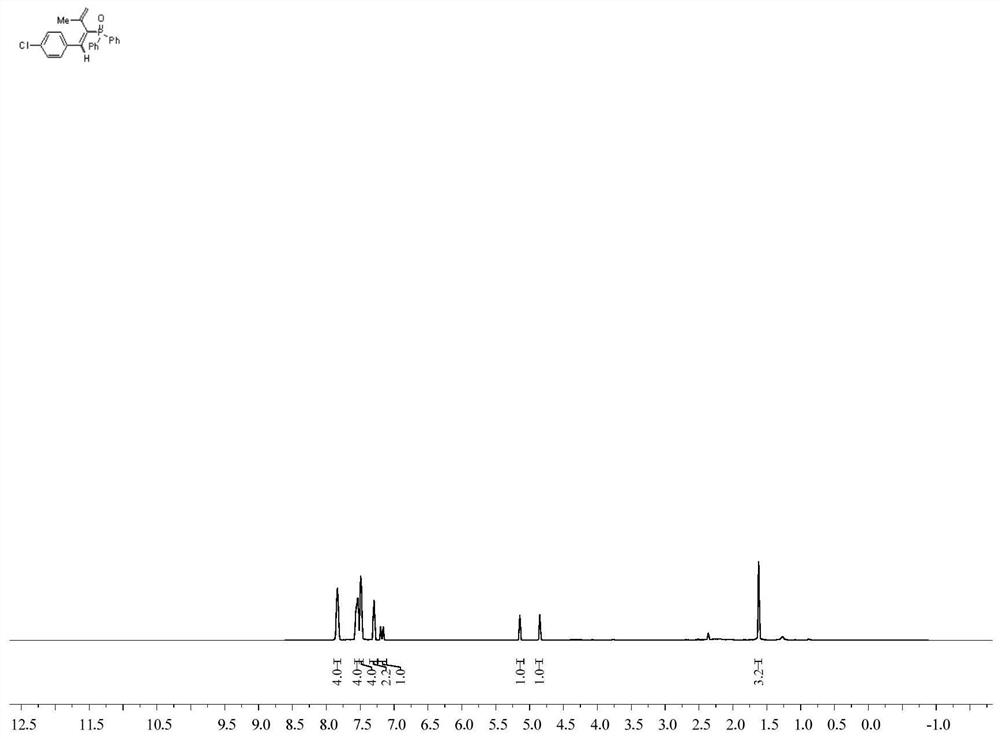
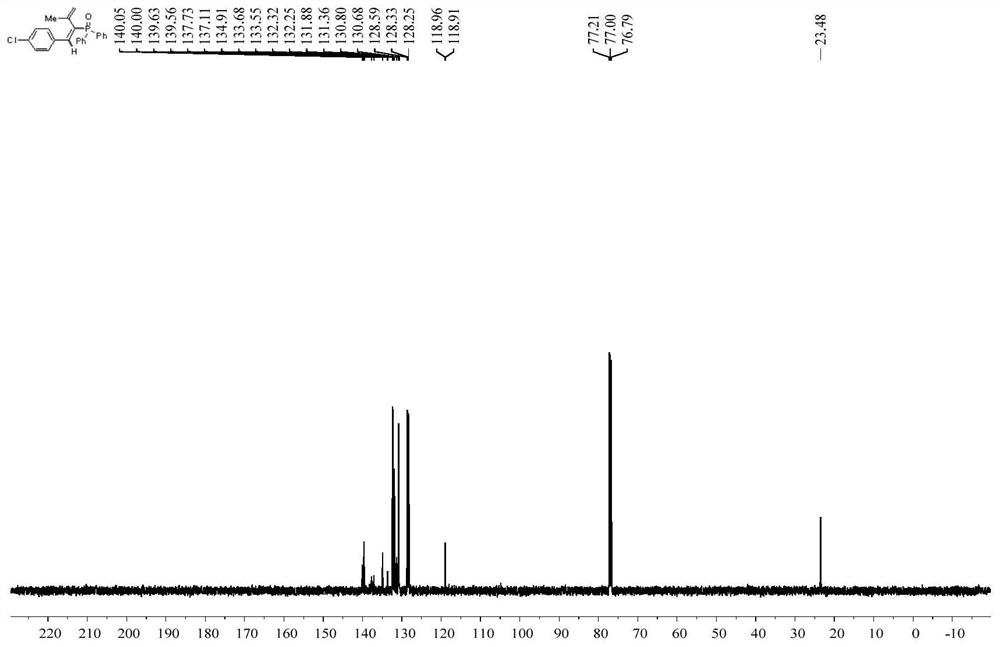
[0033] Weigh 40.44mg (0.2mmol) diphenylphosphine oxide, 13.1mg (0.02mmol) Ni(PPh 3 ) 2 Cl 2 , 52.8mg (0.3mmol) 1-chloro-4-(3-methylbut-3-en-1-ynyl) benzene, 130.3mg (0.4mmol) Cs 2 CO 3 , 4.76mg (0.024mmol) of 1,10-phenanthroline was put into a reaction flask, and 2mL of methanol solution was added, and placed under the irradiation of a 23W blue LED lamp under the protection of argon, the reaction was stirred at room temperature, and the progress of the reaction was detected by TLC. After about 24 hours, it was detected that the reaction was over, and the reacted solution was separated and purified by column chromatography (petroleum ether: ethyl acetate = 3:1) to obtain a white liquid, which was the product (E)-(1-(4-chlorobenzene yl)-3-methylbut-1,3-dienyl)diphenylphosphine oxide, yield: 75%.
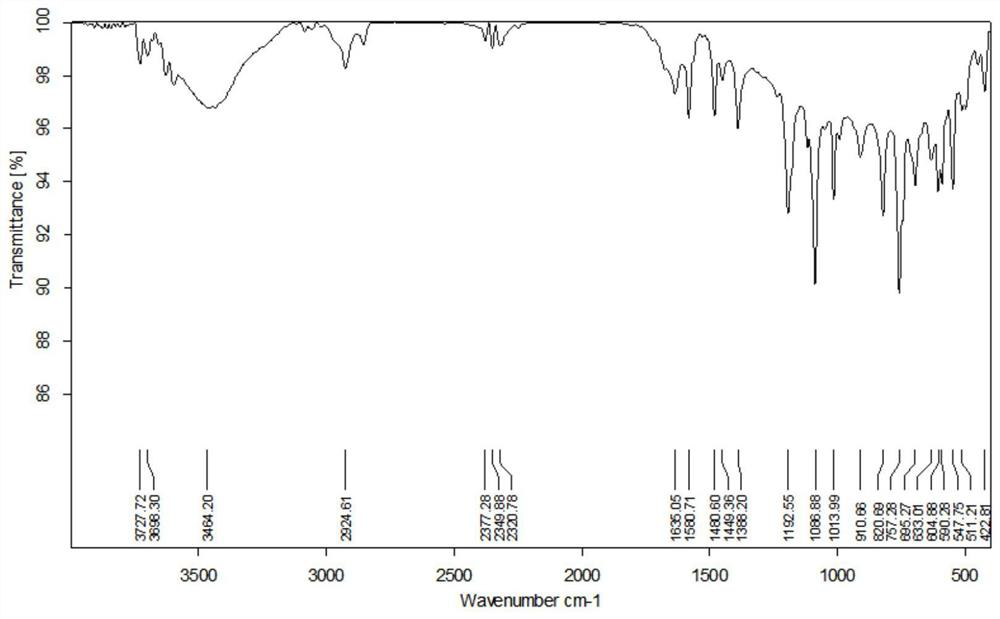
[0034] The product is carried out infrared test, the result is as follows figure 1 Shown, IR (neat): ν = 3059, 2925, 2857, 2218, 1487, 1437, 1187, 1108, 705, 555cm -1 .
[0035]...
Embodiment 2
[0041] Embodiment 2: the impact of different solvents on product yield
[0042] Reaction condition is with embodiment 1, and solvent adopts any one in dichloroethane (DCE), acetonitrile (MeCN), methyl alcohol (MeOH) and acetone (Acetone) respectively, and product yield is shown in table 1 below:
[0043] The impact of different solvents in table 1 on product yield
[0044] Solvent (2mL) DCE Acetone MeOH MeCN Yield(%) less than 5 No reaction 75 25
[0045] As can be seen from Table 1, when other reaction conditions remain unchanged and the solvent is methanol, the highest product yield is 75%, indicating that methanol has good selectivity for this reaction.
Embodiment 3
[0046] Embodiment 3: the impact of different photocatalysts on product yield
[0047] Reaction condition is with embodiment 1, uses respectively Ni (PPh 3 ) 2 Cl 2 , Ni(PPh 3 ) 2 Br 2 、NiBr 2 and NiCl 2 Any one of them, the product yields are shown in Table 2 below.
[0048] Table 2 Effect of different photocatalysts on product yield
[0049] Catalyst (0.02mmol) Ni(PPh 3 ) 2 Br 2
[0050] It can be seen from Table 2 that when other reaction conditions remain unchanged, only Ni(PPh 3 ) 2 Cl 2 Products are produced, and the productive rate is 75%, indicating that Ni(PPh 3 ) 2 Br 2 There is good selectivity for this reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com