Method for purifying contrast agent ioversol
A purification method, ioversol technology, applied in chemical instruments and methods, separation methods, carboxylic acid amide separation/purification, etc., can solve the problems of low overall yield of ioversol, further improvement, harsh purification conditions, etc. , to achieve the effects of easy production process amplification, large-scale industrial production, and short cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The raw materials and equipment used in the present invention are all known products, all of which are obtained by purchasing commercially available products. The preparation of embodiment 1 ioversol pure product of the present invention
[0043] 1. Preparation of ioversol reaction solution
[0044]
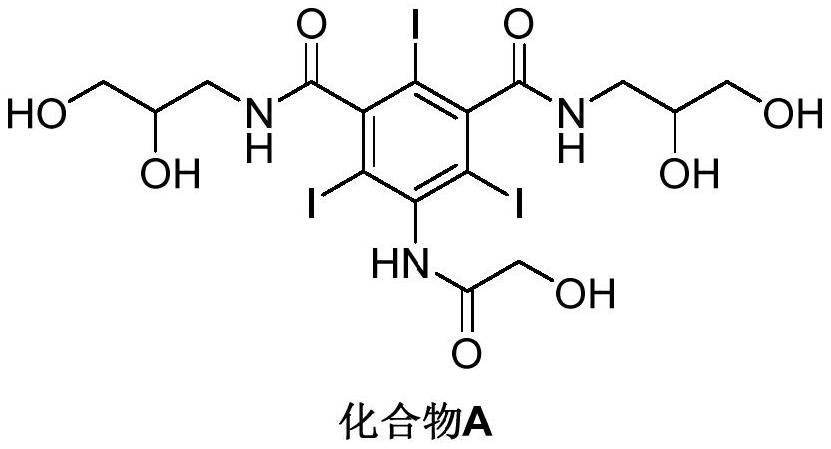
[0045] Add N,N'-bis(2,3-dihydroxypropyl)-5-hydroxyacetamido-2,4,6-triiodoisophthalamide (Compound A, 10.00g) into a 100mL three-necked flask, purified water (30mL), 30% NaOH (3.15g, 1.8eq), after stirring and dissolving, add 2-chloroethanol (1.59g, 1.5eq), heat up to 40~50℃ and react for 4~5 hours, then add hydrochloric acid (1.5mL , concentration 36%~38%) to quench the reaction and adjust the pH to about 6.0~7.0, filter under reduced pressure through filter paper, and obtain the filtrate which is the quenched ioversol reaction solution. The yield is 85%~90%.
[0046] The obtained ioversol reaction solution was analyzed by high performance liquid chromatography (HPLC...
experiment example 1
[0050] Experimental example 1, the purity and related substance analysis of ioversol prepared by the present invention
[0051] 1. Experimental method
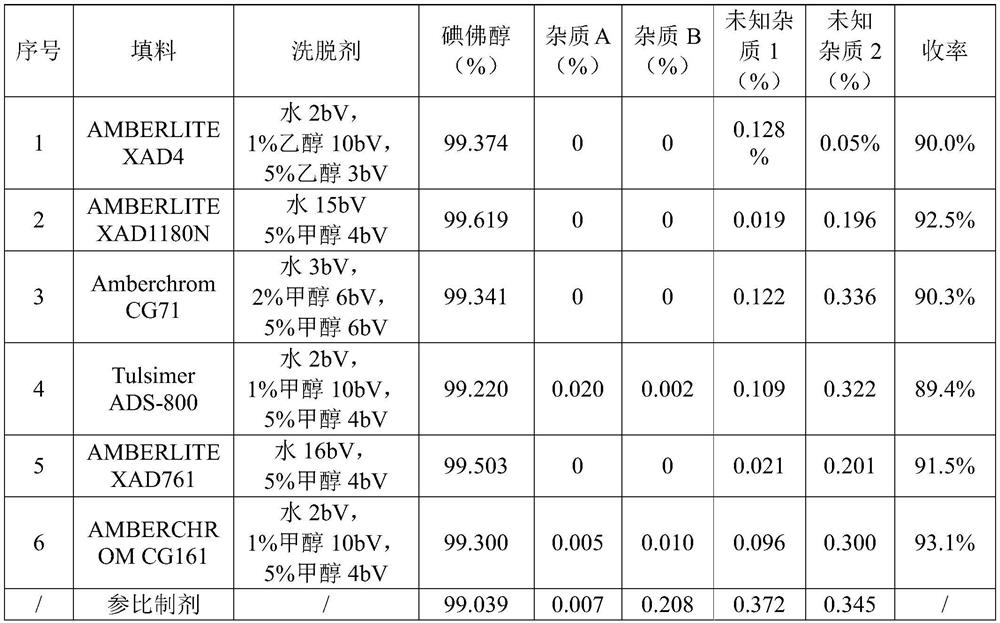
[0052] According to the preparation method of pure ioversol in Example 1, the only difference is that the filler and eluent of the macroporous adsorption resin in step 2 are replaced with the parameters shown in Table 1 to obtain each pure ioversol solution.
[0053] Then get above-mentioned each ioversol pure product solution and carry out HPLC analysis (according to Chinese Pharmacopoeia (2015 edition) in ioversol bulk drug method detection), with reference preparation (ioversol injection (Ansheli) Liebel-Flarsheim CompanyLLC, batch number J250A) is the control, and the results are shown in Table 1.
[0054] 2. Experimental results
[0055] Table 1 Process parameters and product test results of the purification of macroporous adsorption resin
[0056]
[0057] Note: in table 1, impurity A (%), impurity B (%), unknown i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com