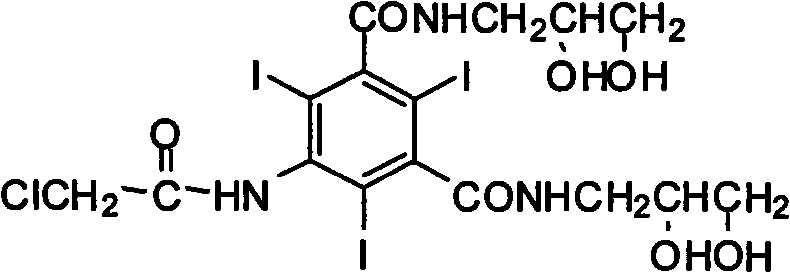
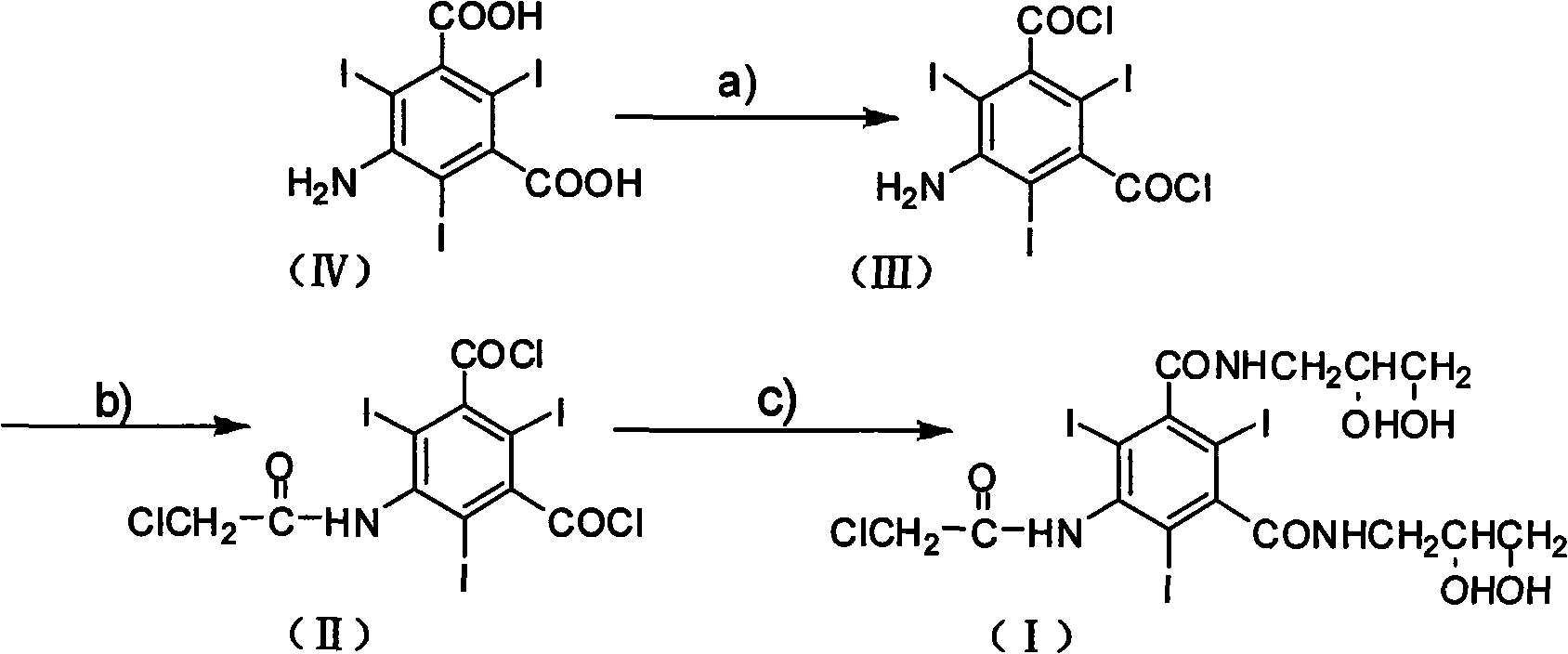
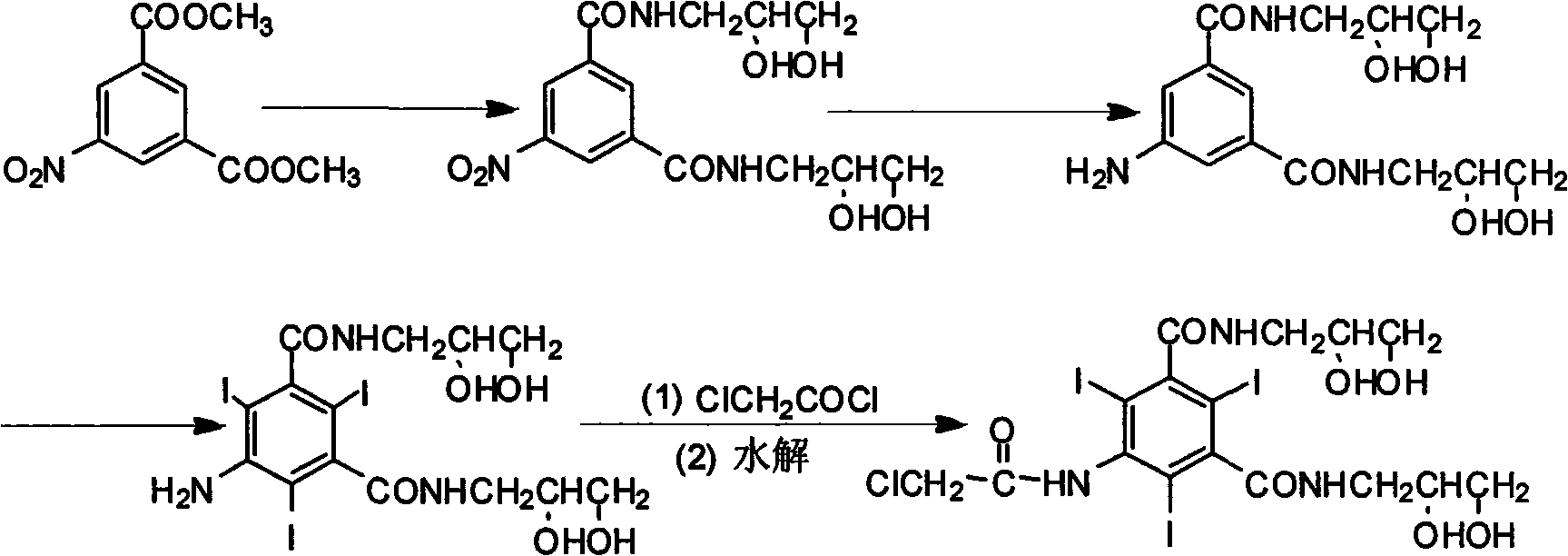
Preparation method of X-ray contrast agent ioversol intermediate
A technology of contrast agent and ioversol, applied in the field of organic compound preparation, can solve the problems of increasing production cost, increasing the dosage of chloroacetyl chloride, etc., and achieves the effects of shortening reaction steps, reducing dosage and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Synthesis of 5-amino-2,4,6-triiodo-1,3-phthaloyl chloride:
Embodiment 2
[0018] Synthesis of 5-chloroacetamido-2,4,6-triiodo-1,3-phthaloyl chloride:
[0019] Dissolve the 5-amino-2,4,6-triiodo-1,3-phthaloyl chloride obtained in the previous step (Example 1) in 125 mL of N,N-dimethylacetamide at room temperature, and stir until uniform Then, cool to 10°C. 16 g (0.15 mol) of chloroacetyl chloride was added dropwise within 30 minutes, then the temperature was raised to 50° C., and the reaction was completed with stirring for 3 hours. After cooling to room temperature, the reaction solution was added dropwise to 300 mL of ice water, during which the temperature was lower than 5 ° C. After stirring for 1 hour, it was filtered, washed with distilled water, and dried to obtain 60 g of the product with a yield of 89% (using 5-amino-2, 4,6-triiodo-1,3-phthalic acid), m.p.>300°C.
Embodiment 3
[0021] Synthesis of 5-chloroacetamido-N, N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide:
[0022] Dissolve 67g (0.1mol) of 5-chloroacetamido-2,4,6-triiodo-1,3-phthaloyl chloride in N,N-dimethylacetamide at room temperature, and cool to below 10°C Finally, 20g (0.2mol) triethylamine and 19g (0.21mol) 3-amino-1,2-propanediol were added, then heated to a reaction temperature of 50°C, stirred for 12 hours to complete the reaction, cooled to below 10°C and filtered. After the filtrate was evaporated under reduced pressure, the residue was dissolved in 60mL (2.5mol / L) sodium hydroxide aqueous solution, and then the pH value was adjusted to 3 with dilute hydrochloric acid, and the precipitated solid was filtered, washed with water, and dried to obtain 71g of the product, which was collected The rate is 91%, m.p.>300°C. 1 H-NMR (DMSO-d 6 , 500MHz) δ (ppm): 3.42 ~ 3.76 (m, 8H), 3.85 (s, 2H), 3.93 ~ 3.96 (m, 2H); MS (FAB) m / z (%): 786 (M + + Na, 100).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com